Epidemiology and Ecology
‹View Table of Contents
West Nile virus (WNV), a mosquito-transmitted member of the genus Flavivirus is the most frequent cause of arboviral disease in the continental United States and is recognized as the most widely distributed arbovirus in the world (Kramer et al. 2008). First identified in northwest Uganda in 1937 (Smithburn et al. 1940), WNV was not viewed as a public health threat until it was associated with epidemics of fever and encephalitis in the Middle East in the 1950s (Taylor et al. 1956). WNV caused only sporadic outbreaks of human disease globally until the mid-1990s, when frequent outbreaks began to occur in the Mediterranean Basin and large outbreaks in Romania and the Volga delta in southern Russia (Hayes et al. 2005).
The first domestically acquired human cases of WNV disease in the Western Hemisphere were detected in New York City in 1999 (Nash et al. 2001). WNV rapidly spread during the following years and by 2005 had established sustained transmission foci in much of the hemisphere with an overall distribution that extended from central Canada to southern Argentina (Gubler 2007).
WNV disease cases have been reported from all 48 contiguous states and two-thirds of U.S. counties. During the first 10 years after WNV was first detected in the United States in 1999, the annual incidence of neuroinvasive disease fluctuated considerably. However, during more recent years, the national incidence of neuroinvasive disease has been relatively stable at around 0.44 per 100,000 population (McDonald et al. 2021). Despite this stability, the occurrence of WNV disease cases continues to be focal and sporadic in nature when assessed at the state and county levels. Annual incidence of WNV disease is most often high in the West Central and Mountain regions, with the highest cumulative incidence of infection in the central plains states (i.e., South Dakota, Wyoming, and North Dakota) (Petersen et al. 2012, McDonald et al. 2021). The greatest disease burden occurs where areas of moderate to high incidence intersect metropolitan counties with high human population densities.
Human WNV disease cases have occurred every month of the year in the United States. However, transmission is highest in summer and early fall, with 94% of human cases reported from July through September and approximately two-thirds of cases in a 6-week period from mid-July through the end of August (McDonald et al. 2021). Weather, especially temperature, is an important modifier of WNV transmission, and has been correlated with increased incidence of human disease at regional and national scales (Soverow et al. 2009).
WNV is primarily maintained in an enzootic transmission cycle between Culex species mosquitoes and birds as the vertebrate hosts. Epidemic (and epizootic) transmission occurs when the virus escapes the bird-to-bird enzootic cycle to infect other vertebrates, including humans. In the US, WNV is enzootic in all 48 contiguous United States and evidence of transmission in the form of infected humans, mosquitoes, birds, horses, or other mammals has been reported from 96% of U.S. counties. Though WNV has been detected in 65 different mosquito species in the United States (CDC 2021), only a few Culex species drive epizootic and epidemic transmission. The most important vectors are Cx. pipiens in the northern states, Cx. quinquefasciatus in the southern states, and Cx. tarsalis in the western states where it overlaps with the Cx. pipiens and Cx. quinquefasciatus (Figure) (Andreadis et al. 2004, Kilpatrick et al. 2006a, Godsey et al. 2012).
Across middle latitudes of the United States, Cx. pipiens and Cx. quinquefasciatus are present both as nominal species and hybrids and are commonly reported as Cx. pipiens complex mosquitoes (Savage and Kothera 2012). Culex salinarius is an important enzootic and epidemic vector in the northeastern United States (Anderson et al. 2004, 2012, Molaei et al. 2006). Other mosquito species including Cx. restuans, Cx. nigripalpus, and Cx. stigmatosoma may contribute to early season amplification or serve as bridge vectors, feeding on both birds and mammals and potentially contributing to human infection (Kilpatrick et al. 2005).
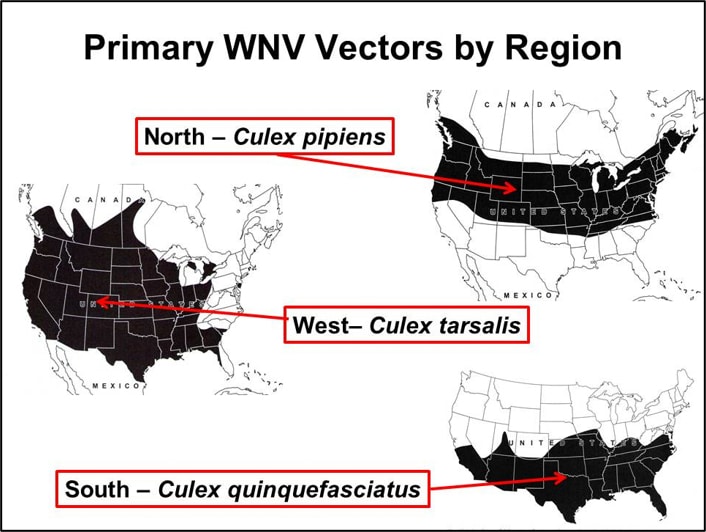
Figure. Approximate geographic distribution of the primary WNV vectors, Cx. pipiens, Cx. quinquefasciatus, and Cx. tarsalis (modified from Darsie and Ward 2005).
WNV has been detected in hundreds of bird species in the United States (CDC 2021) but only a few are primary amplifiers of the virus and influence WNV transmission locally (Hamer et al. 2009). Passerine birds typically are involved in West Nile virus amplification in many locations. For example, the American robin (Turdus migratorious) can be an amplifier host even in locations where it is present in low abundance (Kilpatrick et al. 2006b). Some infected birds, especially crows and jays, are known to get sick and die from the infection.
References
Anderson JF, Andreadis TG, Main AJ, Kline DL. 2004. Prevalence of West Nile virus in tree canopy-inhabiting Culex pipiens and associated mosquitoes. Am J Trop Med Hyg. 71(1):112-9.
Anderson JF, Main AJ, Cheng G, Ferrandino FJ, Fikrig E. 2012. Horizontal and vertical transmission of West Nile virus genotype NY99 by Culex salinarius and genotypes NY99 and WN02 by Culex tarsalis. Am J Trop Med Hyg. 86(1):134-9.
Andreadis TG, Anderson JF, Vossbrinck CR, et al. 2004. Epidemiology of West Nile virus in Connecticut: A five-year analysis of mosquito data 1999–2003. Vector-Borne Zoonotic Dis. 4:360–378.
CDC. 2021. West Nile virus: Mosquito Control. Key Resources for Professionals. https://www.cdc.gov/westnile/vectorcontrol/index.html. Accessed 7/14/2021.
Darsie RF, Ward RA. 2005. Identification and Geographical Distribution of the Mosquitoes of North America, North of Mexico. University of Florida Press, Gainesville, FL. 383 pp.
Godsey MS Jr., Burkhalter K, Young G, Delorey M, Smith K, Townsend J, Levy C, Mutebi JP. 2012. Entomologic investigations during an outbreak of West Nile virus disease in Maricopa County, Arizona, 2010. Am J Trop Med Hyg. 87(6):1125-1131.
Gubler, DJ. 2007. The Continuing Spread of West Nile Virus in the Western Hemisphere. Clinical Inf Dis. 45:1039–46 51
Hamer GL, Kitron UD, Goldberg TL, Brawn JD, Loss SR, Ruiz OR, et al. 2009. Host selection by Culex pipiens mosquitoes and West Nile virus amplification. Am J Trop Med Hyg. 80:268–278.
Hayes EB, Komar N, Nasci RS, Montgomery SP, O’Leary DR, Campbell GL, 2005. Epidemiology and transmission dynamics of West Nile virus disease. Emerg Infect Dis. 11: 1167–1173.
Kilpatrick AM, Kramer LD, Campbell SR, Alleyne EO, Dobson AP, Daszak P. 2005. West Nile virus risk assessment and the bridge vector paradigm. Emerg Infect Dis. 11(3):425-9.
Kilpatrick AM, Kramer LD, Jones MJ, Marra PP, Daszak P. 2006a. West Nile virus epidemics in North America are driven by shifts in mosquito feeding behavior. PLoS Biol. 4:606–610.
Kilpatrick AM, Daszak P, Jones MJ, Marra PP, Kramer LD. 2006b. Host heterogeneity dominates West Nile virus transmission. Proc Biol Sci. 273(1599):2327-33.
Kramer, LD, LM. Styer,GD. Ebel. 2008. A Global Perspective on the Epidemiology of West Nile Virus. Ann Rev Entomol. 53:61–81
McDonald ES, Mathis S, Martin SW, Staples JE, Fischer M, Lindsey NP. 2021.Surveillance for West Nile virus disease — United States, 2009‒2018. MMWR Morb Mortal Wkly Rep;70(1):1-15.
Molaei G, Andreadis TG, Armstrong PM, Anderson JF, Vossbrinck CR. 2006. Host feeding patterns of Culex mosquitoes and West Nile virus transmission, northeastern United States. Emerg Infect Dis. 12(3):468-74.
Nash DMF, Fine A, Miller J, O’Leary D, Murray K, Huang A. 2001. The outbreak of West Nile virus infection in the New York City area in 1999. N Engl J Med. 344: 1807–1814.
Petersen LR, Carson PJ, Biggerstaff BJ, Custer B, Borchardt SM, Busch MP. 2012. Estimated cumulative incidence of West Nile virus infection in US adults, 1999-2010. Epidemiol Infect. 1-5.
Savage HM, Kothera L. 2012. The Culex pipiens complex in the Mississippi River basin: identification, distribution, and bloodmeal hosts. J Am Mosq Control Assoc. 28(4 Suppl):93-9.
Smithburn KC, Hughes TP, Burke AW, Paul JH.1940. A neurotropic virus isolated from the blood of a native of Uganda. Am J Trop Med Hyg. 20:471-92.
Soverow JE, Wellenius GA, Fisman DN, Mittleman MA. 2009. Infectious disease in a warming world: how weather influenced West Nile virus in the United States (2001-2005). Environmental Health Perspect. 117(7):1049-1052.
Taylor RM, Work TH, Hurlbut HS, Rizk F. 1956. A study of the ecology of West Nile virus in Egypt. Am J Trop Med Hyg. 5:579-620. 58
Table of Contents
- About These Guidelines
- ›Epidemiology and Ecology
- Human Disease
- Objectives of Surveillance
- Human Surveillance
- Environmental Surveillance
- ArboNET
- Human Laboratory Diagnosis and Testing
- Non-human Laboratory Diagnosis
- Prevention and Control: Integrated Vector Management
- Prevention and Control: Community Engagement
- Appendix 1: Calculation and Application of a Vector Index (VI) Reflecting the Number of West Nile Virus Infected Mosquitoes in a Population
- Appendix 2: Interim Guidance for States Conducting Avian Mortality Surveillance for West Nile Virus (WNV) or Highly Pathogenic H5N1 Avian Influenza Virus