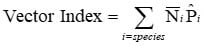Environmental Surveillance
‹View Table of Contents
Vector surveillance is an integral component of an Integrated Vector Management (IVM) program and is the primary tool for quantifying virus transmission and human risk. The principal functions of a mosquito-based surveillance program are to:
- Collect data on mosquito population abundance and virus infection rates in those populations.
- Provide indicators of the threat of human infection and identify geographic areas of high-risk.
- Support decisions regarding the need for and timing of intervention activities (e.g. enhanced vector surveillance and control, use of new technologies and public education programs).
- Monitor the effectiveness of vector control methods, including susceptibility of target mosquitoes to control methods used.
Mosquito-based arboviral monitoring complements disease surveillance programs by contributing fast results and data for action. Programs maintaining in-house laboratories can process mosquito samples daily, giving results within a few days. Data on vector species community composition, relative abundance, and infection rates allow programs to rapidly compute infection indices, assess risk and respond. Maintaining mosquito surveillance over the long-term provides a baseline of historical data to evaluate risk and guide mosquito control operations. However, the utility of mosquito-based surveillance depends both on the type and quality of data collected (e.g., number and type of traps, timing and frequency of sampling, testing procedures) and consistent effort across transmission seasons to link surveillance indices with human risk.
There are three main categories of vector surveillance: larval, adult, and transmission activity. Together, this information is used to determine where and when control efforts should be implemented. Larval surveillance involves sampling a wide range of aquatic habitats to identify the sources of vector mosquitoes and evaluating larval control measures applied. For adult mosquitoes, regular (e.g. monthly, weekly) sampling is done at fixed sites throughout the community that are representative of the habitat types present in the area. Adult mosquitoes are collected using a variety of trapping techniques, including traps for host-seeking, resting, or gravid (carrying eggs) mosquitoes seeking a place to lay eggs (oviposition site).
Light traps collect a wide range of mosquito species (McCardle et al. 2004), providing information about both primary and secondary vectors and a better understanding of the species composition in an area. The three major WNV and St. Louis encephalitis vectors (Cx. pipiens, Cx. quinquefasciatus, and Cx. tarsalis) can be collected in light traps. However, light traps may collect fewer Cx. pipiens or Cx. quinquefasciatus resulting in small sample sizes and less accurate estimates of WNV infection rates.
CDC miniature light traps (Sudia and Chamberlain 1962) are lightweight and use batteries to provide power to a light source and fan motor. CO2 (usually dry ice) is frequently used as an additional attractant. Light traps have several considerations:
- Collections may consist largely of unfed, nulliparous individuals, which greatly reduces the likelihood of detecting WNV and other arboviruses.
- Not all mosquito species are attracted to light traps and the numbers captured may not reflect the population size of a particular species.
- For day-time active mosquitoes other trapping methods should be considered (CDC 2016).
Gravid traps can be useful for sampling Cx. pipiens and Cx. quinquefasciatus, particularly in urban areas (Andreadis and Armstrong 2007, Reisen et al. 1999). Because gravid females have previously taken a blood meal, this increases the likelihood of capturing infected mosquitoes and detecting virus. Gravid traps can be baited with attractants such as fresh or dry grass clipping infusions, rabbit chow infusions, cow manure, fish oil, or other materials that mimic the stagnant water in habitats where these species lay eggs. These vary in attractiveness depending on the type of infusion and its preparation (Burkett et al. 2004, Lampman et al. 1996). Gravid traps mainly capture mosquitoes in the Cx. pipiens complex, and therefore provide limited information on overall species composition within a region (Reiter et al. 1986).
Collecting resting mosquitoes provides a good representation of vector population structure and underlying WNV infection rates, since unfed, gravid, and blood-fed females (as well as males) may be collected. Resting mosquitoes can be collected using suction traps such as the CDC resting trap (Panella et al. 2011), and by using handheld or backpack mechanical aspirators (Nasci 1981) to remove mosquitoes from natural resting harborages or artificial resting structures (e.g., wooden resting boxes, red boxes, fiber pots, and other similar containers). Because of the wide variety of resting sites and the low density of resting mosquitoes in most locations, sampling resting populations is labor intensive and sufficient sample sizes are often difficult to obtain.
Host-baited traps, often employing chickens or pigeons as bait, can collect large numbers of mosquitoes of interest. However, these methods require live animals and adherence to animal use requirements and permitting. The bait species and variations in individual host attractiveness can impact trap performance. These traps target host-seeking mosquitoes and therefore collect mainly unfed, nulliparous individuals.
Human landing collections may expose collectors to infected mosquitoes and are not recommended as a sampling procedure in areas where WNV transmission is occurring.
Since mosquito-based surveillance relies on identifying virus in the collected mosquitoes through detection of viral proteins, viral RNA, or live virus (see Laboratory Diagnosis and Testing section), specimens should be handled in a way that minimizes exposure to conditions (e.g., heat, successive freeze-thaw cycles) that would degrade the virus. Optimally, a cold chain should be maintained from the time mosquitoes are removed from the traps to the time they are delivered to the processing laboratory. Mosquitoes can be transported from the field in a cooler with cold packs or on dry ice, and then placed on a chill-table, if available, during sorting identification, and pooling. Usually only female mosquitoes are tested in routine arboviral surveillance programs. If virus screening is not done immediately after mosquito identification and pooling, the pooled samples should be stored frozen (e.g. -70°C) or at temperatures below freezing for short-term storage. Lack of a cold chain does not appear to reduce the ability to detect viral RNA by reverse transcription polymerase chain reaction (RT-PCR) for WNV (Turell et al. 2002).
Data derived from mosquito surveillance include estimates of mosquito species abundance and infection rate in those mosquito populations. The indices derived from those data vary in information content, ability to be compared over time and space, and association with transmission levels and levels of human risk. Five indicators that have commonly been used: vector abundance, number of positive pools, percent of pools positive, infection rate, and vector index. (Table)
Vector abundance provides a measure of the relative number of mosquitoes in an area during a particular sampling period. It is the total number of mosquitoes of a particular species collected, divided by the number of trapping nights during a specified sampling period, and is expressed as the number/trap night. Risk assessments often consider mosquito abundance because high mosquito densities can be associated with arboviral disease outbreaks (Olson et al. 1979, Eldridge 2004). For example, during a WNV outbreak in Maricopa County, Ariz., 2010, Cx. quinquefasciatus densities were higher in outbreak compared to non-outbreak areas (Godsey et al. 2012, Colborn et al. 2013). However, high mosquito abundance can occur in the absence of virus and outbreaks can occur when abundance is low, but the vector infection rate is high. Vector abundance measures are also used for planning IVM and monitoring the outcomes of mosquito control. Number of traps, their distribution, and the timing of sample collection should be sufficient to obtain spatially and temporally representative data.
Number of positive pools is the total of the number of arbovirus positive mosquito pools detected in a given surveillance location and period. These may be a tally of the total positive pools separated by species or for all species tested. This indicator provides evidence of WNV activity but is not recommended as a stand-alone indicator. Instead, data can be used to produce more informative indices (i.e., Infection Rate and Vector Index).
Percent of pools positive is calculated by the number of positive pools divided by the total number of pools tested, as a percentage. It provides a rough estimate of the rate of infection and can be used to compare activity over time and place. However, the comparative value is limited unless the number of pools tested is large and the number of mosquitoes per pool remains constant. As with the number of positive pools index, these data can be used for the (more informative) Infection Rate and Vector Index.
The Infection Rate in a vector population estimates the prevalence of infected mosquitoes in the population and is a good indicator of human risk. It provides a useful, quantitative basis for comparison, allowing evaluation of changes in infection rate over time and space. Infection rate indices have been used successfully to link infection rates with human risk (Bell et al. 2005). Variable pool numbers and pool sizes can be used, while retaining comparability, but larger sample sizes improve accuracy. Two methods are commonly used to calculate infection rate:
- Minimum infection rate (MIR) for a given mosquito species is the number of positive pools divided by the total number of mosquitoes tested. MIR assumes that infection rates are low and that only one mosquito is positive in a positive pool. MIR is usually expressed as the number infected/1000 tested. It can also be expressed as a proportion or percent positive.
- Maximum likelihood estimate (MLE) is the preferred method, particularly during outbreaks. MLE does not assume only one positive mosquito per positive pool and provides a more accurate estimate when infection rates are high (Gu et al. 2008). The MLE and MIR are similar when infection rates are low. The MLE requires more complex calculations than the MIR; however, a Microsoft Excel® Add-In to compute infection rates from pooled data is available (https://www.cdc.gov/westnile/resourcepages/mosqSurvSoft.html).
The Vector Index (VI) estimates the abundance of infected mosquitoes in an area and incorporates into a single index information on presence, relative abundance, and infection rates of individual species (Gujral et al. 2007, Bolling et al. 2009, Jones et al. 2011). The VI is calculated by multiplying the average number of mosquitoes collected per trap night by the proportion infected. VI is expressed as the average number of infected mosquitoes collected per trap night in the area during the sampling period. In areas with multiple vector species, a VI is calculated for each species. Individual VIs are summed to give a combined estimate of infected vector abundance.
Increases in VI reflect increased risk of human disease and are more reliable prediction measures than vector abundance or infection rate alone (Bolling et al. 2009, Jones et al. 2011, Kwan et al. 2012, Colborn et al. 2013). As with other surveillance indicators, the accuracy of the VI depends on the number of trap nights used to estimate abundance and the number of specimens tested to estimate infection rate. Instructions for calculating the VI in a system with multiple vector species are in Appendix 1.
Use of Vector-based Surveillance Indicators
Mosquito-based surveillance indicators have two important roles in arboviral surveillance and response programs. First, they can provide quantifiable thresholds for proactive vector control efforts. By identifying thresholds for vector abundance and infection rate that are below levels associated with disease outbreaks, IVM programs can institute proactive measures to maintain mosquito populations at levels below which virus amplification can occur. Second, if thresholds related to outbreak levels of transmission can be identified, surveillance can help determine when proactive measures were insufficient to dampen virus amplification and more aggressive measures, such as wide-scale aerial application of mosquito adulticides and expanded public messaging, are needed to stop an outbreak.
| Index | Description | Equation |
|---|---|---|
| Vector Abundance | Number of mosquitoes of a particular vector species captured per trap per night | Number of a particular mosquito species captured in a night/Number of traps set up that night |
| Number of Positive Mosquito Pools | Number of positive mosquito pools detected in a given period of time | Simple count of positive mosquito pools |
| Percentage of Positive Mosquito Pools | Proportion of positive mosquito pools | Number of positive mosquito pools/Total number of pools tested X 100 |
| Infection Rate | An estimate of the number of mosquitoes infected per 1000 tested | Minimum Infection Rate (MIR) = Number of positive pools/Total number of mosquitoes tested
Maximum likelihood estimate (MLE), use links in the footnote. |
| Vector Index | An estimate of the abundance of infected mosquitoes in an area |
 = Number of mosquitoes per trap night for a given species  = Estimated Infection Rate
|
For MLE computations use the mosquito surveillance software at https://www.cdc.gov/westnile/resourcepages/mosqSurvSoft.html
References
Andreadis TG, Armstrong PM. 2007. A two-year evaluation of elevated canopy trapping for Culex mosquitoes and West Nile virus in an operational surveillance program in the Northeastern United States. J Am Mosq Control Assoc. 23(2):137-148.
Bell JA, Mickelson NJ, Vaughan JA. 2005. West Nile virus in host-seeking mosquitoes within a residential neighborhood in Grand Forks. North Dakota Vector-Borne Zoonotic Dis. 5:373
Bolling BG, Barker CM, Moore CG, Pape WJ, Eisen L. 2009. Modeling/GIS, risk assessment, economic impact: Seasonal patterns for entomological measures of risk for exposure to Culex vectors and West Nile virus in relation to human disease cases in Northeastern Colorado. J Med Entomol. 46:1519-1531.
Burkett DA, Kelly R, Porter CH, Wirtz RA. 2004. Commercial mosquito trap and gravid trap oviposition media evaluation, Atlanta, Georgia. J Am Mosq Control Assoc. 20(3): 223-228.
CDC. 2016. Surveillance and Control of Aedes aegypti and Aedes albopictus in the United States. https://www.cdc.gov/mosquitoes/mosquito-control/professionals/index.html (accessed 08/31/2021)
Colborn, J.M., K.A. Smith, J. Townsend, D. Damian, R.S. Nasci, J.P. Mutebi. 2013. West Nile Virus Outbreak in Phoenix, Arizona—2010: Entomological Observations and Epidemiological Correlations. J Amer Mosq Control Assoc. 29(2):123-32.
Eldridge BF. 2004. Surveillance for arthropodborne diseases. In Eldridge and Edman eds. Medical Entomology, Kluwer Academic Press, Dordrecht, The Netherlands, pp 645
Godsey MS Jr., Burkhalter K, Young G, Delorey M, Smith K, Townsend J, Levy C, Mutebi JP. 2012. Entomologic investigations during an outbreak of West Nile virus disease in Maricopa County, Arizona, 2010. Am J Trop Med Hyg. 87(6):1125-1131.
Gu W, Unnasch TR, Katholi CR, Lampman R, Novak RJ. 2008. Fundamental issues in mosquito surveillance for arboviral transmission. Trans R Soc Trop Med Hyg. 102: 817-822.
Gujral IB, Zielinski-Gutierrez EC, LeBailly A, Nasci R. 2007. Behavioral risks for West Nile virus disease, northern Colorado, 2003. Emerg Infect Dis. 13(3):419-25.
Jones RC, Weaver KN, Smith S, Blanco C, Flores C, Gibbs K, Markowski D, Mutebi JP. 2011. Use of the vector index and geographic information system to prospectively inform West Nile virus interventions. J Am Mosq Control Assoc. 27:315-319.
Kwan JL, Park BK, Carpenter TE, Ngo V, Civen R, Reisen WK. 2012. Comparison of enzootic risk measures for predicting West Nile disease, Los Angeles, California, USA, 2004-2010. Emerg Infect Dis. 18(8):1298-306.
Lampman RL, Novak RJ. 1996. Oviposition preferences of Culex pipiens and Culex restuans for infusion-baited traps. J Am Mosq Control Assoc. 12(1):23-32.
McCardle PW, Webb RE, Norden BB, Aldrich JR., 2004. Evaluation of five trapping systems for the surveillance of gravid mosquitoes in Prince Georges County, Maryland. J Am Mosq Control Assoc. 20(3):254-260.
Nasci RS. 1981. A lightweight battery-powered aspirator for collecting resting mosquitoes in the field. Mosq News. 41: 808-811.
Olson JG, Reeves WC, Emmons RW, Milby MM. 1979. Correlation of Culex tarsalis population indices with the incidence of St. Louis encephalitis and western equine encephalomyelitis in California. Am J Trop Med Hyg. 28: 335-343.
Panella NA, Crockett RJ, Biggerstaff BJ, Komar N. 2011. The Centers for Disease Control and Prevention resting trap: a novel device for collecting resting mosquitoes. J Am Mosq Control Assoc. 27(3):323-325.
Reisen WK, Boyce K, Cummings RC, Delgado O, Gutierrez A, Meyer RP, Scott TW. 1999. Comparative effectiveness of three adult mosquito sampling methods in habitats representative of four different biomes of California. J Med Entomol. 36(1):23-29.
Reiter P, Jakob WL, Francy DB, Mullenix JB. 1986. Evaluation of the CDC gravid trap for the surveillance of St. Louis encephalitis vectors in Memphis, Tennessee. J Am Mosq Control Assoc. 2(2):209-211.
Sudia WD, Chamberlain RW. 1962. Battery-operated light trap an improved model. Mosq News, 22: 126-129.
Turell MJ, Spring AR, Miller MK, Cannon CE. 2002. Effect of holding conditions on the detection of West Nile viral RNA by reverse transcriptase-polymerase chain reaction from mosquito (Diptera: Culicidae) pools. J Med Entomol. 39(1):1-3.
Animal-based Surveillance
WNV amplifies in nature by replicating to high levels in a variety of bird species (326 affected species reported to ArboNET through 2016; CDC 2016), which then transmit the virus to mosquitoes during several days of sustained high-level viremia. In addition to infection from mosquito bites, some birds are infected by consuming infected prey (insects, small mammals, other birds) or in rare cases, from direct contact with other infected birds. A hallmark of the North American strain of WNV is its propensity to kill many birds it infects. Corvids (species of the family Corvidae, including crows, ravens, magpies, and jays) and other select species are particularly susceptible (Komar 2003). Avian morbidity/mortality surveillance and monitoring infections in wild or captive birds are strategies used to determine WNV activity and can provide a quantitative index of risk for human infection.
Dead bird reporting systems collect broad information about the temporal and spatial patterns of bird deaths in an area and provide insight into WNV activity. Public participation is essential and must be encouraged through an effective public education and outreach program. A system for carcass reporting should be established including a database to record and analyze dead bird sightings with the following suggested data: caller identification and call-back number, date observed, location geocoded to the highest feasible resolution, species, and condition. A subset of the reported bird deaths can be investigated to confirm WNV activity. Birds in good condition (not scavenged and without obvious decomposition or maggot infestation) may be sampled or retrieved for laboratory testing (see Avian morbidity/mortality testing). Dead bird reporting systems provide a wide surveillance net extending to any area where a person is present to observe a dead bird. These systems have been used with success to estimate risk of human infection (Eidson et al. 2001a, Mostashari et al. 2003, Carney et al. 2011).
There are several limitations to dead bird surveillance systems. Maintaining public interest and willingness to participate is essential to these programs but is difficult to maintain. The surveillance is passive and qualitative and can only be used to assess risk of infection to people in areas where sufficient data are collected to populate risk models such as DYCAST (Carney et al. 2011) and SaTScan (Mostashari et al. 2003). Over time, bird populations can become resistant to morbidity and mortality (Reed et al. 2009), compromising the utility of this surveillance for WNV. Other causes of bird mortality could cause a false alarm for WNV activity, although this might also alert the public health and wildlife disease communities to other pathogens or health threats.
In programs where the objective of avian morbidity/mortality testing is early detection of WNV activity and not a quantitative index of human risk, testing dead birds should be initiated when local adult mosquito activity begins in the spring, and continue as long as local WNV activity is undetected in the area. Once WNV is detected in dead birds, or if vector prevention and control actions have been initiated, continued detection of WNV in carcasses in that area does not provide additional information about WNV activity and is not necessary or cost-effective. However, the number of WNV-infected dead birds can contribute to an effective human risk index (Kwan et al. 2012a).
Contact with WNV-infected carcasses presents a potential health hazard to handlers (Fonseca et al. 2005). Appropriate biosafety precautions should be taken when handling carcasses in the field and in the laboratory. More detailed guidelines for sampling avian carcasses are available in Appendix 2.
To maximize sensitivity of this surveillance system, a variety of bird species should be tested, but corvids should be emphasized if they are present (Nemeth et al. 2007a). In dead corvids and other birds, bloody pulp from immature feathers, and tissues collected at necropsy such as brain, heart, kidney, or skin harbor very high viral loads, and any of these specimen types is sufficient for sensitive detection of WNV (Panella et al. 2001, Komar et al. 2002, Docherty et al. 2004, Nemeth et al. 2009, Johnson et al. 2010). Oral swabs and breast feathers are easy specimens to collect in the field, avoid the need to transfer dead birds to the laboratory, do not require a cold chain, and are effective for detecting WNV in dead corvids (Komar et al. 2002, Nemeth et al. 2009). They are less sensitive for WNV detection in non-corvids; however, the reduced sensitivity of testing non-corvids using these tissue types can be offset by sampling more carcasses. The number of bird specimens tested will be dependent upon resources and whether WNV-infected birds have already been found in the area; triage of specimens by species or by geographic location may be appropriate in some jurisdictions.
Several studies have demonstrated the effectiveness of avian mortality testing for early detection of WNV activity (Eidson et al. 2001b, Julian et al. 2002, Guptill et al. 2003, Nemeth et al. 2007b, Patnaik et al. 2007, Kwan et al. 2012a). Wildlife rehabilitation clinics can be a good source of specimens derived from carcasses (Nemeth et al. 2007b). Collecting samples from living birds that are showing signs of illness requires the assistance of a veterinarian or wildlife technician. Dead crows and raptors alarm the public and carcasses are easily spotted. However, in regions with few or no crows, carcasses may be less obvious. Eye aspirates have been shown to be a sensitive and fast sampling protocol for WNV detection in corvid carcasses brought to the laboratory for testing (Lim et al 2009).
The use of living birds as sentinels for monitoring WNV transmission requires serially blood-sampling a statistically valid number of avian hosts. Captive chickens, frequently referred to as sentinel chickens, (though other species have been used) provide the most convenient source of blood for this purpose. Blood may be collected from a wing vein, the jugular vein, or on Nobuto® strips by pricking the chicken’s comb with a lancet. There is no standard protocol for implementing a sentinel chicken program. It can be tailored to the specific circumstances of each surveillance jurisdiction, though sentinel chicken systems generally employ flocks of 6-10 birds at each site and bleed each bird weekly or every other week throughout the WNV transmission season. Sentinel chicken-based WNV surveillance systems can provide evidence of WNV transmission several weeks in advance of human cases (Healy et al. 2012).
While serially sampling free-ranging bird species is very labor intensive, it can provide information about seroconversion in amplifier hosts, similar to the data provided by sentinel chickens. Quantifying seroprevalence in free-ranging birds may provide additional information that benefits surveillance programs (Komar 2001). For example, a serosurvey of the local resident bird population (in particular, juvenile birds) following the arbovirus transmission season may help determine which local species may be important amplifiers of WNV in the surveillance area. This in turn could be used to map areas of greatest risk in relation to the populations of amplifier hosts. Furthermore, a serosurvey of adult birds just prior to arbovirus transmission season can detect pre-existing levels of antibody in the bird population. High levels would suggest less opportunity for WNV amplification because many adult bird species transfer maternal antibodies to their offspring, which can delay or inhibit WNV amplification among the population of juvenile birds that emerges each summer. In Los Angeles, California, serosurveys of local amplifier hosts during winter determined that subsequent outbreaks occurred only after seroprevalence dipped below 10% in these birds (Kwan et al. 2012b).
There are several advantages of sentinel chicken and other live-bird serology surveillance systems. Sentinel chickens are captive, so a seroconversion event indicates local transmission and presence of infected mosquitoes in the area. Chickens do not develop clinical disease, nor do they develop viremias sufficient to infect mosquitoes (Langevin et al. 2001). Chickens are preferred blood-feeding hosts of Cx. pipiens and Cx. quinquefasciatus, which are important urban vectors of WNV. Chickens can be used to monitor seroconversions of multiple arboviruses of public health importance (i.e., WNV, SLE, WEE, and EEE viruses) simultaneously. However, there are also a number of important limitations related to these systems. Determination that a chicken has seroconverted occurs typically 3-4 weeks after the transmission event has occurred and reporting of a positive chicken may not precede the first local case of human disease caused by WNV (Patnaik et al. 2007, Kwan et al. 2010, Unlu et al. 2009). Use of sentinel birds requires institutional animal use and care protocols, and other authorization permits. Linking patterns in sentinel chicken seroconversion with human risk requires multiple years of data.
Horses are susceptible to encephalitis due to WNV infection; thus, equine cases of WNV-induced encephalitis may serve a sentinel function in the absence of other environmental surveillance programs. Equine health is an important economic issue, so severe disease in horses comes to the attention of the veterinary community. Use of horses as sentinels for active WNV surveillance is theoretically possible, but practically infeasible. Widespread use of equine WNV vaccines decreases the incidence of equine WNV disease, and survivors of natural infections are protected from disease, reducing the usefulness of equines as sentinels. Veterinarians, veterinary service societies/agencies, and state agriculture departments are essential partners in any surveillance activities involving WNV infections in horses. Equine disease due to WNV is rare in tropical ecosystems. However, WNV frequently infects horses in the tropics. Detection of seroconversions in horses has been suggested as a sentinel system to detect risk of WNV transmission to people in Puerto Rico and other tropical locations (Phoutrides et al. 2011, Mattar et al. 2011).
Small numbers of other mammal species have been affected by WNV. Dead squirrels are tested for WNV along with dead birds in some jurisdictions. Among domestic mammals, the most important has been the camelids, such as llamas and alpacas. As with horses, these come to the attention of veterinarians and any veterinary case of disease due to WNV may be used for passive surveillance. Dogs and cats become infected with WNV. Active surveillance of WNV in dogs has been shown to predict human infection with WNV (Resnick et al. 2008). WNV disease in dogs is rare and vaccination of dogs has not been recommended or practiced. Maintaining a large number of seronegative dogs for use as sentinels would be cumbersome, but juvenile stray dogs could be used for this purpose in areas where other surveillance methods are not available. Stray dog removal programs could provide a source of samples at low cost. WNV infects cats but cats have not been evaluated as surveillance sentinels. There is no evidence that dogs or cats develop sufficient viremia to become amplifier hosts (Austgen et al. 2004).
References
Austgen LE, Bowen RA, Bunning ML, Davis BS, Mitchell CJ, Chang GJ. 2004. Experimental infection of cats and dogs with West Nile virus. Emerg Inf Dis. 10(1)82-86.
Carney RM, Ahearn SC, McConchie A, Glasner C, Jean C, Barker C, Park B, Padgett K, Parker E, Aquino E,Kramer V. 2011. Early warning system for West Nile virus risk areas, California, USA. Emerg Infect Dis. 7(8):1445-54.
CDC. 2016. Species of dead birds in which West Nile virus has been detected, United States, 1999-2016. https://www.cdc.gov/westnile/resources/pdfs/BirdSpecies1999-2016.pdf [PDF – 4 pages]
Docherty DE, Long RR, Griffin KM, Saito EK. 2004. Corvidae feather pulp and West Nile virus detection. Emerg Infect Dis. 10(5):907-9.
Eidson M. 2001a. “Neon needles” in a haystack: the advantages of passive surveillance for West Nile virus. Ann N Y Acad Sci. 951:38-53.
Eidson M, Schmit K, Hagiwara Y, Anand M, Backenson PB, Gotham I. 2001b. Dead crow densities and human cases of West Nile virus, New York State. Emerg Infect Dis. 7:662–4.
Fonseca K, Prince GD, Bratvold J, Fox JD, Pybus M, Preksaitis JK, Tilley P. 2005. West Nile virus infection and conjunctival exposure. Emerg Infect Dis. 11(10):1648-9.
Guptill SC, Julian KG, Campbell GL, Price SD, Marfin AA. 2003. Early-season avian deaths from West Nile virus as warnings of human infection. Emerg Infect Dis. 9(4):483-4.
Healy J, Reisen WK, Kramer V, Barker CM. 2012. Do current surveillance methods provide adequate warning for human infections with West Nile virus? Proc Mosq Control Assoc Calif. 80:17-21.
Johnson G, Nemeth N, Hale K, Lindsey N, Panella NA, Komar N. 2010. Surveillance for West Nile virus in American white pelicans, Montana, USA, 2006-2007. Emerg Infect Dis. 16(3):406–11.
Julian KG, Eidson M, Kipp AM, Weiss E, Petersen LR, Miller JR, Hinten SR, Marfin AA. 2002. Early season crow mortality as a sentinel for West Nile virus disease in humans, northeastern United States. Vector Borne Zoonotic Dis. 2(3):145-55.
Komar N. 2001. West Nile virus surveillance using sentinel birds. Annals NY Acad Sci. 951:58-73.
Komar N, Lanciotti R, Bowen R, Langevin S, Bunning M. 2002. Detection of West Nile virus in oral and cloacal swabs collected from bird carcasses. Emerg Infect Dis. 8(7):741-2.
Komar N. 2003. West Nile virus: epidemiology and ecology in North America. Adv. Vir. Res. 61:185-234.
Kwan JL, Kluh S, Madon MB, Nguyen DV, Barker CM, Reisen WK. 2010. Sentinel chicken seroconversions track tangential transmission of West Nile virus to humans in the greater Los Angeles area of California. Am J Trop Med Hyg. 83(5):1137-45.
Kwan JL, Park BK, Carpenter TE, Ngo V, Civen R, Reisen WK. 2012a. Comparison of enzootic risk measures for predicting West Nile disease, Los Angeles, California, USA, 2004-2010. Emerg Infect Dis. 18(8):1298-306.
Kwan JL, Kluh S, Reisen WK. 2012b. Antecedent avian immunity limits tangential transmission of West Nile virus to humans. PLoS One. 7(3):e34127.
Langevin SA, Bunning M, Davis B, Komar N. 2001. Experimental infection of chickens as candidate sentinels for West Nile virus. Emerg Infect Dis 7(4):726-729.
Lim AK, Dunne G, Gurfield N. 2009. Rapid bilateral intraocular cocktail sampling method for West Nile virus detection in dead corvids. J Vet Diagn Invest. 21(4):516-9.
Mattar S, Komar N, Young G, Alvarez J, Gonzalez M. 2011. Seroconversion to West Nile and St. Louis encephalitis viruses among sentinel horses, Colombia. Memorias do Instituto Oswaldo Cruz. 106(8): 976-79.
Mostashari F, Kulldorff M, Hartman JJ, Miller JR, Kulasekera V. 2003. Dead bird clusters as an early warning system for West Nile virus activity. Emerg Infect Dis. 9:641-646. Doi: 10.3201/eid0906.020794
Nemeth N, Beckett S, Edwards E, Klenk K, Komar N. 2007a. Avian mortality surveillance for West Nile virus in Colorado. Am J Trop Med Hyg. 76:431-7.
Nemeth N, Kratz G, Edward E, Scherpelz J, Bowen R, Komar N. 2007b. Evaluation of clinic-admitted raptors for West Nile virus surveillance. Emerg Infect Dis. 13(2): 305-307.
Nemeth NM, Burkhalter KL, Young GR, Brault AC, Reisen WK, Komar N. 2009. West Nile virus detection in nonvascular feathers from avian carcasses. J Vet Diagn Invest. 21:616–622.
Panella NA, Kerst AJ, Lanciotti RS, Bryant P, Wolf B, Komar N. 2001. Comparative West Nile virus detection in organs of naturally infected American crows (Corvus brachyrhynchos). Emerg Infect Dis. 7(4):754-5.
Patnaik JL, Juliusson L, Vogt RL. 2007. Environmental predictors of human West Nile virus infections, Colorado. Emerg Infect Dis. 13(11):1788-90.
Phoutrides E, Jusino-Mendez T, Perez-Medina T, Seda-Lozada R, Garcia-Negron M, Davila-Toro F, Hunsperger E. 2011. The utility of animal surveillance in the detection of West Nile virus activity in Puerto Rico, 2007. Vector Borne Zoonotic Dis. 11(4):447-50.
Reed LM, Johannson MJ, Panella N, McLean RG, Creekmore T, Puelle R, Komar N. 2009. Declining mortality in American crow (Corvus brachyrhynchos) following natural West Nile virus infection. Avian Dis. 53:458–61.
Resnick MP, Grunenwald P, Blackmar D, Hailey C, Bueno R, Murray KO. 2008. Juvenile dogs as potential sentinels for West Nile virus surveillance. Zoonoses Public Health. 55(8-10):443-7
Unlu I, Roy AF, Yates M, Garrett D, Bell H, Harden T, Foil LD. 2009. Evaluation of surveillance methods for detection of West Nile virus activity in East Baton Rouge Parish, Louisiana, 2004-2006. J Am Mosq Control Assoc. 25(2):126-33. Doi:10.2987/08-5713.1
Table of Contents
- About These Guidelines
- Epidemiology and Ecology
- Human Disease
- Objectives of Surveillance
- Human Surveillance
- ›Environmental Surveillance
- ArboNET
- Human Laboratory Diagnosis and Testing
- Non-human Laboratory Diagnosis
- Prevention and Control: Integrated Vector Management
- Prevention and Control: Community Engagement
- Appendix 1: Calculation and Application of a Vector Index (VI) Reflecting the Number of West Nile Virus Infected Mosquitoes in a Population
- Appendix 2: Interim Guidance for States Conducting Avian Mortality Surveillance for West Nile Virus (WNV) or Highly Pathogenic H5N1 Avian Influenza Virus