Chlamydia Screening Among Females Aged 15–21 Years — Multiple Data Sources, United States, 1999–2010
Corresponding author: Karen W. Hoover, Division of STD Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, CDC. Telephone: 404-639-8534; Email: ffw6@cdc.gov.
Introduction
Chlamydia is a sexually transmitted infection caused by the bacterium Chlamydia trachomatis. Chlamydia is the most commonly reported notifiable disease in the United States, with 1.4 million cases reported in 2012 (1). Chlamydia is usually asymptomatic in both men and women, and as a result, infections often are undiagnosed. Approximately 3 million new infections are estimated to occur each year (1). Among sexually active females aged 14–19 years, chlamydia prevalence has been estimated to be 6.8% (2). In a recent study involving approximately 1 million tests conducted among both privately insured and Medicaid-insured females aged 15–21 years, chlamydia positivity ranged from 6.9% to 10.7% among those with chlamydial symptoms and from 6.1% to 9.6% among those who were asymptomatic (3).
Chlamydial infection in females causes cervicitis, which is usually asymptomatic; however, infection sometimes can cause such symptoms as abnormal vaginal discharge, intermenstrual bleeding, dyspareunia, dysuria, or pelvic pain (4). If chlamydial cervicitis is untreated, infection can ascend to the upper genital tract along with other microorganisms that are part of the vaginal microflora (e.g., anaerobic bacteria) to cause pelvic inflammatory disease (PID) (5,6). The inflammatory and immune responses induced during PID can cause fallopian tube damage, scarring, and blockage (7), which can result in long-term adverse outcomes of tubal factor infertility, ectopic pregnancy, and chronic pelvic pain (8). Some females who have uncomplicated cervicitis have concurrent subclinical PID at the time that chlamydial cervicitis is diagnosed (9), and subclinical PID also has been associated with infertility (10). Screening for and treating chlamydial infection decreases incidence of PID (11,12). Screening for chlamydia also identifies persons at increased risk for HIV infection. Chlamydial infection has been linked to HIV transmission (13,14) because of similar sexual risk behaviors and possible increased biologic susceptibility caused by breaches in the mucosa and inflammation from the infection. Chlamydial infection is diagnosed easily by using vaginal or endocervical swab specimens or urine samples that are tested with nucleic acid amplification tests (NAATs), and treatment with oral antibiotics is simple, safe, and effective (4).
Routine annual chlamydia screening of sexually active young women is one of several important preventive reproductive health-care services. The U.S. Preventive Services Task Force (USPSTF) recommends annual chlamydia screening of all sexually active nonpregnant females aged ≤24 years. This is a USPSTF Grade A recommendation, which means that USPSTF strongly recommends the service. The certainty is high that the benefits of screening for chlamydial infection substantially outweigh the harms. USPSTF concluded that the harms of screening for chlamydia infection are no greater than small, although few studies have been published on this subject (15). USPSTF also recommends screening of pregnant females aged ≤24 years. This is a USPSTF Grade B recommendation, which means that USPSTF recommends the service. The certainty is moderate that the benefits substantially outweigh the harms of screening for chlamydia infection (15). Healthy People 2020 objectives include increasing the proportion of sexually active females aged 16–24 years who are screened each year for genital chlamydial infection (objectives STD-3 and STD-4) (16). The National Quality Forum (NQF) has endorsed as a performance measure the percentage of sexually active females aged 16–24 years who had at least one test for chlamydia in a year (NQF #0033) (17).
Public health has an essential role in monitoring adherence to recommendations for chlamydia screening and in working with the health-care sector and other stakeholders to develop and implement interventions to increase screening. Monitoring screening coverage of young women has been challenging. Screening refers to testing of asymptomatic persons, but no single available data source provides a valid, accurate, and reliable estimate of chlamydia screening coverage in sexually active asymptomatic young women. Because chlamydial infections usually are asymptomatic, chlamydia testing rates have been used to estimate screening rates.
The reports in this supplement provide the public and stakeholders responsible for infant, child, and adolescent health (including public health practitioners, parents or guardians and their employers, health plans, health professionals, schools, child care facilities, community groups, and voluntary associations) with easily understood and transparent information about the use of selected clinical preventive services that can improve the health of infants, children, and adolescents. The topic in this report is one of 11 topics selected on the basis of existing evidence-based clinical practice recommendations or guidelines for the preventive services and availability of data system(s) for monitoring (18). This report analyzes 1999–2010 data from multiple data sources to estimate the prevalence of chlamydia screening among U.S. females aged 15–21 years. Public health authorities and clinicians can use these data to identify population subgroups that might require additional strategies to increase access and utilization of chlamydia screening.
Methods
Multiple data sources can be used to assess chlamydia testing coverage and provide insight into chlamydia screening patterns. The National Survey of Family Growth (NSFG) and the National Ambulatory Medical Care Survey (NAMCS) can be used to estimate population-based testing coverage of adolescent females and their use of chlamydia testing at visits to physician offices. NSFG provides estimates of self-reported testing and uses the ideal denominator of an estimate of all U.S. females who reported sexual activity. NAMCS provides insight into use patterns of this reproductive health service among females who have access to health care and identifies missed opportunities for screening of U.S. females who had clinical encounters. Healthcare Effectiveness Data and Information Set (HEDIS) measures of chlamydia testing of women enrolled in commercial and Medicaid health plans, and Title X-funded family planning clinic testing data can be used to monitor temporal trends in chlamydia screening.
To estimate chlamydia screening rates of U.S. adolescent females, CDC used four data sources: the 2006–2010 NSFG, the 2005–2010 NAMCS, 1999–2010 HEDIS measure data, and 2005–2010 Title X data. The age range of 15–21 years was used for analyses with NSFG and NAMCS data; HEDIS data were available only for females aged 16–20 years and Title X data only for those aged 15–19 years.
To estimate the percentage of sexually active females aged 15–21 years who reported that they had been tested for chlamydia in the past 12 months, CDC analyzed 2006–2010 NSFG data. NSFG is a multistage national probability sample of the U.S. population aged 15–44 years residing in households (19). During 2006–2010, a total of 12,279 females were interviewed, and the response rate was 78% (20). The survey methods were similar to those described previously (21). Analyses were limited to the 1,811 sexually active females aged 15–21 years who reported that they had been tested for chlamydia in the past 12 months. Being sexually active was defined as reporting having had one or more male sex partners in the past 12 months; and having sex included having vaginal intercourse, oral sex, or anal sex. The frequency of chlamydia testing was estimated by the females' demographic characteristics, self-reported number of sexual partners in the past 12 months, and self-reported receipt of reproductive health-care services in the past 12 months. Data were weighted to provide nationally representative estimates, and analytic methods were used to account for the complex sampling procedure used by NSFG. Differences between percentages of females were compared by using the Chi-square test; a two-sided p-value <0.05 was considered statistically significant.
To estimate the mean annual percentage of nonpregnant females aged 15–21 years who were tested for chlamydia at visits to primary care physician offices, CDC analyzed NAMCS data from 2005–2010. Primary care specialties included general and family practice, internal medicine, pediatrics, and obstetrics and gynecology. NAMCS is a multistage national probability sample of visits to nonfederally employed U.S. physician offices, including private practices and other freestanding clinics (e.g., urgent care centers, public health clinics, family planning clinics, mental health centers, community health centers, and faculty practice plans) (22). The unit of analysis used was a patient visit, with extraction of data from a review of the patient's medical record. In 2007, medical records from 32,778 patient visits to 1,568 physicians were reviewed, with a response rate of 61% (22). Survey methods used were similar to those described previously (23–25); CDC estimated the frequency of chlamydia testing at visits made by nonpregnant females aged 15–21 years by their demographic characteristics, primary care provider specialty, diagnosis at visit, and receipt of reproductive health-care service. Data were weighted to provide nationally representative estimates, and analytic methods were used to account for the complex sampling procedures used by NAMCS. Differences between percentages of visits were compared by using the Chi-square test; a two-sided p-value <0.05 was considered statistically significant.
Since 1999, the National Committee for Quality Assurance (NCQA) has collected health-care claims data for HEDIS measures of annual chlamydia screening among young females. NCQA is a private nonprofit organization that monitors the quality of U.S. health plans using data that are submitted voluntarily to HEDIS annually. HEDIS is used by 90% of U.S. health plans to evaluate the quality of health-care services and to benchmark performance (26). To estimate the annual percentage of chlamydia screening among sexually active females aged 16–20 years enrolled in commercial and Medicaid health-care plans during 1999–2010, CDC used administrative data with International Classification of Diseases 9th Edition and Current Procedural Terminology (CPT) billing codes. Methods used were similar to those described previously (26). Sexually active females were defined as those who had health-care encounters for a gynecologic examination, pregnancy, contraception, sexually transmitted disease (STD) services, cervical cancer screening, or infertility evaluation or treatment. Among females who had one of these encounters, a chlamydia test was identified by using CPT codes for chlamydia testing at the health-care encounter.
Title X is a federal program administered by the U.S. Department of Health and Human Services' Office of Population Affairs (OPA). Title X provides family planning and related preventive health-care services, with priority given to low-income persons; services include chlamydia testing. Since 2005, the program has monitored chlamydia testing, with service grantees reporting testing data to OPA annually (27,28). For purposes of this analysis, family planning clinic users were assumed to be sexually active and therefore should have been screened according to recommendations. For each year during 2005–2010, the percentage of users who were tested for chlamydia was calculated as the number of unduplicated female users aged 15–19 years who were tested among all family planning users in this age group.
Results
On the basis of NSFG data, a weighted estimate of 8.2 million (56.6%) U.S. females aged 15–21 years reported that they were sexually active, of whom 3.30 million (40.0%) reported that they had been tested for chlamydia in the past 12 months (Table). A chlamydia test was reported by a significantly larger proportion of sexually active women aged 20–21 years (50.0%) than by adolescents aged 18–19 years (38.2%) or those aged 15–17 years (25.2%) (p<0.001). Non-Hispanic black adolescent females had the highest testing rates (56.1%) compared with members of other racial and ethnic groups (p<0.001). Females who had Medicaid insurance or were uninsured had higher testing rates (48.4% and 43.8%, respectively) than those who had private insurance (33.8%) (p<0.001). Females with an income-to-poverty ratio of ≤138% had higher testing rates (42.7%) than those with a ratio of >138% (38.0) (p<0.05). Females who had two or more sexual partners had higher testing rates (45.8%) than those who had only one partner (36.3%) (p<0.001). A larger proportion of sexually active females who had received other reproductive health services (e.g., family planning or contraception, a pregnancy test, pelvic examination, or a Papanicolaou test) in the past 12 months reported having had a chlamydia test compared with females who did not receive these services.
On the basis of NAMCS data, among a weighted estimate of 20.9 million visits to primary care physician offices made by females aged 15–21 years, a chlamydia test was performed at only 4.3% of visits (Table). Testing was performed more often at visits to obstetrics and gynecology offices (11.1%) than at visits to other primary care specialties (2.3%; p<0.001). A chlamydia test was very rare at visits to pediatricians, with no chlamydia test performed at most visits (99.1% [standard error: 0.48]). Females who made visits with symptoms or signs of chlamydial infection were tested at 9.9% of these visits, but only at 3.2% of visits for other reasons (p<0.001). Screening was performed at 8.4% of visits for preventive care compared with other visits (2.5%; p<0.001) and more frequently at visits for reproductive health services than at visits for other services. A chlamydia test was performed at 28.1% of visits with a Pap test compared with 1.7% of visits without Pap testing (p<0.001).
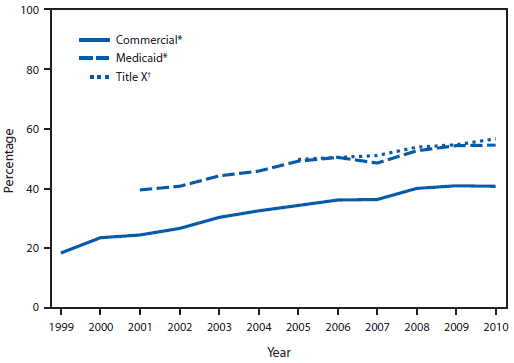
During 1999–2010, the HEDIS measure of chlamydia testing of commercially insured females aged 16–20 years increased from 18.5% to 40.8% (Figure). During 2001–2010, the HEDIS measure of chlamydia testing of Medicaid-insured females aged 16–21 years increased from 39.6% to 54.6%. During 2005–2010, Title X service providers tested 3.4 million female family planning users aged 15–19 years for chlamydia, and the percentage of females tested for chlamydia increased from 49.8% in 2005 to 56.7% in 2010 (Figure). Over all years, the annual rates of chlamydia testing of Medicaid-insured females and Title X female family planning clinic users were both higher compared with rates of commercially insured females.
Discussion
In this report, nationally representative rates of chlamydia screening of U.S. adolescent females were estimated by using NSFG data to generate self-reported testing rates and by using NAMCS data to generate testing rates at visits on the basis of medical record review. HEDIS measure data and Title X data were used to estimate temporal trends in the annual chlamydia testing rate. Although the methods, age groups, and units of measure varied, all the findings support the conclusion that many sexually active adolescent females in the United States were not tested as recommended, even when they visited a physician with symptoms and signs consistent with chlamydial infection.
Chlamydia testing rates were lowest at visits to pediatricians, who conduct 48% of all health-care visits for adolescents aged 15–16 years and 23% of all visits for those aged 17–18 years (29), two age groups with high rates of infection (1–3). Many screening opportunities at clinical visits were missed for young females, including at preventive visits. Preventive visits are an ideal opportunity to discuss sexual and reproductive health issues, including STD and pregnancy prevention, and to perform chlamydia screening (30,31). Testing rates were higher among females who used reproductive health services, both by self-report in NSFG and by medical record review in NAMCS. However, even at visits for reproductive health care, testing was suboptimal, and many opportunities were missed. Testing coverage has not increased to a sufficient extent over the 12-year period that HEDIS data have been monitored. Although testing rates were higher for Medicaid-insured females and Title X family planning clinic users compared with commercially insured females, testing rates in all these groups were suboptimal. Chlamydia screening of young females has been demonstrated to be cost-effective compared with other common clinical preventive services (32). Nevertheless, it is relatively underutilized compared with other preventive services recommended by USPSTF (32).
Ongoing changes in the U.S. health-care system offer opportunities to improve the use of clinical preventive services among infants, children, and adolescents. The Patient Protection and Affordable Care Act of 2010 (as amended by the Health Care and Education Reconciliation Act of 2010 and referred to collectively as the Affordable Care Act [ACA]) expands insurance coverage, consumer protections, and access to care and places a greater emphasis on prevention (33). As of September 23, 2010, ACA § 1001 requires nongrandfathered private health plans to cover, with no cost-sharing, a collection of four types of clinical preventive services, including 1) recommended services of USPSTF graded A (strongly recommended) or B (recommended) (34); 2) vaccinations recommended by the Advisory Committee on Immunization Practices (35); 3) services adopted for infants, children, and adolescents under the Bright Futures guidelines supported by the Health Resources and Services Administration (HRSA) and the American Academy of Pediatrics (36) and those developed by the Discretionary Advisory Committee on Heritable Disorders in Newborns and Children (37); and 4) women's preventive services as provided in comprehensive guidelines supported by HRSA (38). USPSTF recommends chlamydia screening as a Grade A service for sexually active nonpregnant females aged ≤24 years and for older nonpregnant women at increased risk (15). Screening is recommended as a Grade B service for pregnant females aged ≤24 years (15). State Medicaid programs cover chlamydia screening as part of the Early and Periodic Screening, Diagnostic and Treatment benefit (39).
The Health Insurance Marketplace (or Health Insurance Exchange) began providing access to private health insurance for small employers and to persons and families interested in exploring their options for coverage, with policies taking effect as early as January 2014.* Federal tax credits are available on a sliding scale to assist those living at 100%–400% of the federal poverty level who purchase health insurance through the Marketplace (ACA § 1401). Insurance plans sold on the Marketplace must cover the four types of recommended clinical preventive services without cost-sharing, including chlamydia screening.
In 2010, a total of 18% of U.S. females aged 15–21 years were uninsured (40,41). Although ACA will decrease barriers to access to chlamydia screening services, it is difficult to anticipate the extent to which use of chlamydia screening will increase. Chlamydia screening has not been used fully by those who currently have access to health care, with low rates of screening at visits for preventive care and reproductive health services. Even as access to services without patient costsharing expands, the challenge of increasing use will remain. Barriers to screening adolescents include lack of provider skill and comfort in taking the sexual history that is required to identify sexually active adolescents who should be screened (42), and lack of disclosure of sexual behavior by adolescents (43). Patients might have concerns about lack of confidentiality caused by an explanation of benefits that is sent by a health plan to the policy holder, who is often the adolescent's parent or guardian (44). Adolescents might not have access to a health-care provider or venue where they think that their privacy would be maintained (45,46). Clinicians or adolescents might not be aware of the risk for infection. Many competing demands and priorities in an often brief clinical encounter also can be a barrier to chlamydia screening, especially given the sensitive discussion about sexual behaviors that is necessary to identify those who should be tested.
CDC, in collaboration with its public health partners, can develop and implement simple, affordable, and sustainable interventions to overcome barriers and facilitate screening of adolescents. These interventions are needed to ensure that as barriers to health care are decreased by ACA, chlamydia screening services are accessible to all sexually active females. Although toolkits and other resources have been developed (31,47,48) and widely disseminated to primary care providers including pediatricians (48,49), screening rates have been low. Possible interventions to increase screening include the use of electronic health record prompts to increase screening and retesting of those who are found positive. Prompts have been demonstrated to be most effective when used as part of a more comprehensive effort that includes additional interventions including implementation teams, training of providers, provider feedback, and panel management (50–52). Structural interventions (e.g., clinic protocols and procedures) can facilitate adherence to recommendations for screening and improve health-care system performance. The structural intervention of placing a chlamydia collection swab beside a cervical cytology kit has been demonstrated to be successful in ensuring that a chlamydia test also was performed (53). However, because cervical cancer screening guidelines no longer recommend cervical cytologic screening for females aged <21 years (54), alternative interventions need to be developed as fewer adolescents are screened for cervical cancer and thus for chlamydia (55). Providers and patients will need to use other existing opportunities to test for chlamydia, such as at visits for preventive care, contraception, and pregnancy testing (56). In addition, the implementation of patient-centered interventions that facilitate self-collection of specimens can be effective to increase screening. Primary care providers might perform chlamydia screening more often if they were to be made aware that a test can be performed easily without a pelvic exam by using a self-collected vaginal swab specimen or urine (4). Finally, social marketing campaigns for young females, such as the Get Yourself Tested (GYT) campaign, have been demonstrated to increase patient demand for screening (57,58).
Limitations
The findings in this report are subject to at least nine limitations. First, although NSFG data are representative of all sexually active U.S. females and accurately identify females who should have been tested on the basis of their self-reported sexual activity, testing rates might be either overestimated or underestimated because females who had a chlamydia test were identified by self-report of the test. Many females who have a gynecologic examination or Pap test might incorrectly assume that a chlamydia test also was performed (59), resulting in an overestimate of testing. Females might not know that a chlamydia test was performed, and this also might lead to an underestimate of testing. Second, with NAMCS data, chlamydia tests are more accurately identified by a review of the patient's medical record, but the unit of analysis is a visit rather than a person. Young females might have had more than one visit in a year and other opportunities for testing besides the reviewed visit, possibly leading to an underestimate of testing rates. In contrast, NSFG captures testing performed in venues in addition to physician offices. Third, NAMCS data do not distinguish whether females are sexually active or not, and nonsexually active females would not require a test. This would result in an underestimate of the testing rate. However, reproductive health visits probably were made by sexually active adolescents, and rates of testing rates at these visits were also very low. Fourth, among the data sources analyzed, only NAMCS included variables to distinguish symptomatic and asymptomatic screening. In contrast, NSFG does not collect data on whether females were symptomatic or asymptomatic for chlamydial infection, so it was not possible to distinguish screening of asymptomatic females from testing of symptomatic females. Fifth, the HEDIS measure of chlamydia screening includes only females who utilize reproductive health-care services, likely resulting in inclusion of too few females in the denominator and an overestimate of testing rates. Sixth, all female family planning clinic users were included in the analysis of Title X data on the assumption that they were all sexually active. Some users might not have been sexually active, and their inclusion in the denominator would result in an underestimate of screening rates. However, it is likely that most adolescents seeking reproductive health care at a Title X service site are sexually active. Seventh, with both HEDIS measures and Title X data, service providers might change over time, and trends in testing might reflect changes in the service providers participating in the program rather than changes within clinics. Eighth, rates calculated using HEDIS and Title X data were made on the basis of convenience samples, so the findings cannot be generalized to the U.S. population. Finally, Medicaid and Title X family planning clinic users might be at increased risk for chlamydial infection (60,61), and this might have led clinicians who care for these populations to be more likely to test for chlamydia, resulting in an overestimate of testing rates compared with the general population of U.S. adolescents.
Conclusion
In the United States, chlamydia screening rates have been suboptimal with fewer than half of sexually active females aged 15–21 years screened annually. Although testing and screening rates varied by demographic characteristics, insurance type, screening venue, and type of health-care services used, suboptimal rates indicate that improvement in screening coverage is needed. Provision of this simple, affordable, effective, and cost-effective service can protect the reproductive health of adolescents and prevent infertility and ectopic pregnancy. Interventions are needed to increase patient and provider adherence to the recommendation for annual chlamydia screening of all sexually active females aged ≤24 years.
CDC will continue to use the four data sources described in this report to monitor chlamydia screening trends. The usefulness of surveys would be enhanced by adding additional questions to ascertain whether a female was symptomatic or sexually active. Together, these data can be used to track trends in chlamydia screening and to provide valuable information for improving access and use of this important preventive service by adolescent females. Access to chlamydia screening will likely be increased with implementation of ACA, and CDC and its public health partners will use the chlamydia testing data provided in this report to develop focused interventions for at-risk groups and to identify missed opportunities for screening and testing of those who access care.
References
- CDC. Sexually transmitted disease surveillance, 2012. Atlanta, GA: U.S. Department of Health and Human Services; 2014.
- CDC. CDC grand rounds: chlamydia prevention: challenges and strategies for reducing disease burden and sequelae. MMWR 2011;60:370–3.
- Hoover KW, Tao G, Nye MB, Body BA. Suboptimal adherence to repeat testing recommendations for men and women with positive chlamydia tests in the United States, 2008–2010. Clin Infect Dis 2013;56:51–7.
- CDC. Sexually transmitted diseases treatment guidelines, 2010. MMWR 2010;59(No. RR-12).
- Mårdh PA, Ripa T, Svensson L, Westrom L. Chlamydia trachomatis infection in patients with acute salpingitis. N Engl J Med 1977;296:1377–9.
- Wasserheit JN, Bell TA, Kiviat NB, et al. Microbial causes of proven pelvic inflammatory disease and efficacy of clindamycin and tobramycin. Ann Intern Med 1986;104:187–93.
- Paavonen J, Westrom L, Eschenbach D. Pelvic inflammatory disease. In: Holmes K, Sparling P, Stamm WE, et al., eds. Sexually transmitted diseases. 4th ed. New York, NY: McGraw-Hill Companies, Inc.; 2008:1017–50.
- Weström L, Joesoef R, Reynolds G, Hagdu A, Thompson SE. Pelvic inflammatory disease and fertility. A cohort study of 1,844 women with laparoscopically verified disease and 657 control women with normal laparoscopic results. Sex Transm Dis 1992;19:185–92.
- Wiesenfeld HC, Hillier SL, Krohn MA, et al. Lower genital tract infection and endometritis: insight into subclinical pelvic inflammatory disease. Obstet Gynecol 2002;100:456–63.
- Wiesenfeld HC, Hillier SL, Meyn LA, Amortegui AJ, Sweet RL. Subclinical pelvic inflammatory disease and infertility. Obstet Gynecol 2012;120:37–43.
- Oakeshott P, Kerry S, Aghaizu A, et al. Randomised controlled trial of screening for Chlamydia trachomatis to prevent pelvic inflammatory disease: the POPI (prevention of pelvic infection) trial. BMJ 2010;340:c1642.
- Scholes D, Stergachis A, Heidrich FE, Andrilla H, Holmes KK, Stamm WE. Prevention of pelvic inflammatory disease by screening for cervical chlamydial infection. N Engl J Med 1996;334:1362–6.
- Fleming DT, Wasserheit JN. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Infect 1999;75:3–17.
- Sexton J, Garnett G, Rottingen JA. Metaanalysis and metaregression in interpreting study variability in the impact of sexually transmitted diseases on susceptibility to HIV infection. Sex Transm Dis 2005;32:351–7.
- US Preventive Services Task Force. Screening for chlamydial infection: US Preventive Services Task Force recommendation statement. Ann Intern Med 2007;147:128–34.
- US Department of Health and Human Services. Healthy people 2020. Topics and objectives. Sexually transmitted diseases. Washington, DC: US Department of Health and Human Services; 2013. Available at http://www.healthypeople.gov/2020/topicsobjectives2020/objectiveslist.aspx?topicId=37.
- National Quality Forum. Chlamydia screening in women. Washington, DC: National Committee for Quality Assurance; 2011. Available at http://www.qualityforum.org/Measures_Reports_Tools.aspx.
- Yeung LF, Shapira SK, Coates RJ, et al. Rationale for periodic reporting on the use of selected clinical preventive services to improve the health of infants, children, and adolescents—United States. In: Use of selected clinical preventive services to improve the health of infants, children, and adolescents —United States, 1999–2011. MMWR 2014;63(No. Suppl 2).
- Lepkowski J M, Mosher WD, Davis KE, Groves RM, Van Hoewyk J. The 2006–2010 National Survey of Family Growth: sample design and analysis of a continuous survey. Vital Health Stat Series 2, Data Evaluation and Methods Research 2010:1–36.
- Chandra A, Billioux VG, Copen CE, Sionean C. HIV risk-related behaviors in the United States household population aged 15–44 years: data from the National Survey of Family Growth, 2002 and 2006–2010. Natl Health Stat Report 2012;1–19 .
- Tao G, Hoover KW, Leichliter JS, Peterman TA, Kent CK. Self-reported Chlamydia testing rates of sexually active women aged 15–25 years in the United States, 2006–2008. Sex Transm Dis 2012;39:605–7.
- Hsiao CJ, Cherry DK, Beatty PC, Rechtsteiner EA. National Ambulatory Medical Care Survey: 2007 summary. Natl Health Stat Report 2010;1–32.
- Eugene JM, Hoover KW, Tao G, Kent CK. Higher yet suboptimal Chlamydia testing rates at community health centers and outpatient clinics compared with physician offices. Am J Public Health 2012;102:e26–9.
- Hoover K, Tao G. Missed opportunities for chlamydia screening of young women in the United States. Obstet Gynecol 2008;111:1097–102.
- Hoover K, Tao G, Kent C. Low rates of both asymptomatic chlamydia screening and diagnostic testing of women in US outpatient clinics. Obstet Gynecol 2008;112:891–8.
- CDC. Chlamydia screening among sexually active young female enrollees of health plans—United States, 2000–2007. MMWR 2009;58:362–5.
- US Department of Health and Human Services, Office of Population Affairs. Family planning annual reports. Washington, DC: US Department of Health and Human Services, Office of Population Affairs; 2014. Available at http://www.hhs.gov/opa/title-x-family-planning/research-and-data/fp-annual-reports.
- US Department of Health and Human Service, Office of Population Affairs. Title X family planning annual report: forms and instructions. Washington, DC: US Department of Health and Human Services, Office of Population Affairs; 2007. Available at http://www.hhs.gov/opa/pdfs/fpar-forms-and-instructions.pdf.
- Hoover KW, Tao G, Berman S, Kent CK. Utilization of health services in physician offices and outpatient clinics by adolescents and young women in the United States: implications for improving access to reproductive health services. J Adolesc Health 2010;46:324–30.
- American College of Obstetricians and Gynecologists. Guidelines for women's health care, a resource manual. 3rd ed. Washington, DC: American College of Obstetricians and Gynecologists; 2007.
- American College of Obstetricians and Gynecologists. Primary and preventive health care for female adolescents. Washington, DC: American College of Obstetricians and Gynecologists; 2010.
- Maciosek MV, Coffield AB, Edwards NM, Flottemesch TJ, Goodman MJ, Solberg LI. Priorities among effective clinical preventive services: results of a systematic review and analysis. Am J Prev Med 2006;31:52–61.
- Patient Protection and Affordable Care Act of 2010. Pub. L. No. 114–148 (March 23, 2010), as amended through May 1, 2010. Available at http://www.healthcare.gov/law/full/index.html.
- US Preventive Services Task Force. USPSTF A and B recommendations. Rockville, MD: US Preventive Services Task Force; 2014. Available at http://www.uspreventiveservicestaskforce.org/uspstf/uspsabrecs.htm.
- CDC. Vaccine recommendations of the Advisory Committee on Immunization Practices, Atlanta, GA: US Department of Health and Human Services, CDC. Available at http://www.cdc.gov/vaccines/hcp/acip-recs/index.html.
- Hagan JF, Shaw JS, Duncan PM, eds. Bright futures: guidelines for health supervision of infants, children, and adolescents. 3rd ed. Elk Grove Village, IL: American Academy of Pediatrics; 2008.
- Health Resources and Services Administration. Discretionary Advisory Committee on Heritable Disorders in Newborns and Children. About the Committee. Rockville, MD: US Department of Health and Human Services, Health Resources and Services Administration. Available at http://www.hrsa.gov/advisorycommittees/mchbadvisory/heritabledisorders/about/index.html.
- Health Resources and Services Administration. Women's preventive services guidelines. Rockville, MD: US Department of Health and Human Services, Health Resources and Services Administration; 2013. Available at http://www.hrsa.gov/womensguidelines.
- Centers for Medicare and Medicaid. Early and periodic screening, diagnostic and treatment. Baltimore, MD: US Department of Health and Human Services, Centers for Medicare and Medicaid Services; 2014. Available at http://www.medicaid.gov/Medicaid-CHIP-Program-Information/By-Topics/Benefits/Early-and-Periodic-Screening-Diagnostic-and-Treatment.html.
- US Census Bureau. Current Population Survey (CPS). Table creator. 2007. Washington, DC: US Department of Commerce, US Census Bureau; 2014. Available at http://www.census.gov/cps/data/cpstablecreator.html.
- Adams PE, Martinez ME, Vickerie JL, Kirzinger WK. Summary health statistics for the U.S. population: National Health Interview Survey. Vital and health statistics Series 10. Data from the National Health Survey 2010; 2011:1–117.
- Huppert JS, Adams-Hillard PJ. Sexually transmitted disease screening in teens. Curr Womens Health Rep 2003;3:451–8.
- DiClemente RJ, Sales JM, Danner F, Crosby RA. Association between sexually transmitted diseases and young adults' self-reported abstinence. Pediatrics 2011;127:208–13.
- Slive L, Cramer R. Health reform and the preservation of confidential health care for young adults. J Law Med Ethics 2012;40:383–90.
- Golden MR, Kerndt PR. Improving Clinical operations: can we and should we save our STD clinics? Sex Transm Dis 2010;37:264–5.
- Felsenstein D. A universal health insurance mandate does not equate to universal coverage for STI clinic patients [Presentation]. National STD Prevention Conference. Minneapolis, Minnesota; March 12–15, 2012.
- American College of Obstetricians and Gynecologists. Sexually transmitted infections in adolescents. In: Guidelines for adolescent health care. 2nd ed. Washington, DC: American College of Obstetricians and Gynecologists; 2011:64–72.
- Society for Adolescent Health and Medicine. Clinical care resources. Dearfield, IL: Society for Adolescent Health and Medicine; 2014. Available at http://www.adolescenthealth.org/Clinical-Care-Resources.aspx.
- American Academy of Pediatrics. Adolescent health. Elk Grove Village, IL: American Academy of Pediatrics; 2012. Available at http://www2.aap.org/sections/adolescenthealth/default.cfm.
- Scholes D, Grothaus L, McClure J, et al. A randomized trial of strategies to increase chlamydia screening in young women. Prev Med 2006;43:343–50.
- Walker J, Fairley CK, Walker SM, et al. Computer reminders for Chlamydia screening in general practice: a randomized controlled trial. Sex Transm Dis 2010;37:445–50.
- Loo TS, Davis RB, Lipsitz LA, et al. Electronic medical record reminders and panel management to improve primary care of elderly patients. Arch Intern Med 2011;171:1552–8.
- Burstein GR, Snyder MH, Conley D, et al. Chlamydia screening in a health plan before and after a national performance measure introduction. Obstet Gynecol 2005;106:327–34.
- American College of Obstetricians and Gynecologists, Committee on Practice. ACOG Practice Bulletin no. 109: Cervical cytology screening. Obstet Gynecol 2009;114:1409–20.
- Tao G, Hoover KW, Kent CK. 2009 cervical cytology guidelines and chlamydia testing among sexually active young women. Obstet Gynecol 2010;116:1319–23.
- Gee RE. Preventive services for women under the affordable care act. Obstet Gynecol 2012;120:12–4.
- McFarlane M. Evaluation of GYT: Get yourself tested, using existing data sources to gather evidence of success [Presentation.] National STD Prevention Conference. Minneapolis; March 12–15, 2012.
- Planned Parenthood Federation of America. GYT09 report and looking ahead. New York, NY: Planned Parenthood Federation of America; 2009.
- Ogbechie OA, Hacker MR, Dodge LE, Patil MM, Ricciotti HA. Confusion regarding cervical cancer screening and chlamydia screening among sexually active young women. Sex Transm Infect 2012;88:35–7.
- Christiansen-Lindquist L, Tao G, Hoover K, Frank R, Kent C. Chlamydia screening of young sexually active, Medicaid-insured women by race and ethnicity, 2002–2005. Sex Transm Dis 2009;36:642–6.
- Satterwhite CL, Grier L, Patzer R, Weinstock H, Howards PP, Kleinbaum D. Chlamydia positivity trends among women attending family planning clinics: United States, 2004–2008. Sex Transm Dis 2011;38:989–94.
* The Health Insurance Marketplace was set up to provide a state-based competitive insurance marketplace. The Marketplace allows eligible persons and small businesses with up to 50 employees (and increasing to 100 employees by 2016) to purchase health insurance plans that meet criteria outlined in ACA (ACA § 1311). If a state did not create a Marketplace, the federal government operates it.
|
TABLE. (Continued) Rate of self-reported chlamydia testing in the past 12 months among sexually active females aged 15–21 years and mean annual rate of chlamydia testing at physician office visits of nonpregnant females aged 15–21 years — National Survey of Family Growth, United States, 2006–2010 and National Ambulatory Medical Care Survey, United States, 2005–2010 |
||||||
|---|---|---|---|---|---|---|
|
Characteristic |
Sexually active females*,† |
Physician office visits† |
||||
|
No. |
% that reported a chlamydia test (SE) |
p value |
No. |
Percentage with a chlamydia test (SE) |
p value |
|
|
Symptomatic††† |
||||||
|
Yes |
NA |
NA |
3,566,590 |
9.86 (1.67) |
<0.001 |
|
|
No |
NA |
NA |
17,370,710 |
3.17 (0.44) |
||
|
Family planning/contraception §§§ |
||||||
|
Yes |
5,223,784 |
47.9 (2.1) |
<0.001 |
1,828,590 |
14.81 (2.35) |
<0.001 |
|
No |
3,018,309 |
26.2 (2.4) |
19,108,700 |
3.31 (0.41) |
||
|
Pregnancy test¶¶¶ |
||||||
|
Yes |
2,845,130 |
61.2 (2.5) |
<0.001 |
1,087,980 |
19.87 (4.46)** |
<0.001 |
|
No |
4,656,040 |
32.5 (2.0) |
20,568,100 |
3.75 (0.47) |
||
|
Urine test |
||||||
|
Yes |
NA |
NA |
2,751,180 |
11.39 (2.05) |
<0.001 |
|
|
No |
NA |
NA |
18,186,120 |
3.24 (0.39) |
||
|
Pelvic exam |
||||||
|
Yes |
3,983,180 |
61.1 (2.1) |
<0.001 |
3,773,780 |
17.73 (1.96) |
<0.001 |
|
No |
4,258,910 |
20.1 (2.0) |
17,163,510 |
1.36 (0.35) |
||
|
Pap test |
||||||
|
Yes |
4,955,090 |
57.2 (2.0) |
<0.001 |
2,041,230 |
28.08 (3.25) |
<0.001 |
|
No |
3,287,000 |
14.0 (1.7) |
18,896,070 |
1.74 (0.34) |
||
|
Total |
8,242,090**** |
40.0 (1.7) |
20,937,300**** |
4.31 (0.44) |
||
|
Abbreviations: NA = not available; NAMCS = National Ambulatory Medical Care Survey; NSFG = National Survey of Family Growth; SCHIP = State Children's Health Insurance Program; SE = standard error. * Females aged 15–21 years who reported having any type of sex with one or more male partners in the past 12 months. † By nonpregnant U.S. females aged 15–21 years; estimates weighted for the probability of selection, nonresponse rate, and population ratio. § Persons of Hispanic ethnicity can be of any race or combination of races. ¶ Includes Asian, Hawaiian/Pacific Islander, American Indian/Alaska Native, and multiple races. ** Estimate might be unstable because it is based on <100 respondents (NSFG) or <30 visits (NAMCS). †† Northeast: Connecticut, Maine, Massachusetts, New Hampshire, New Jersey, New York, Pennsylvania, Rhode Island, and Vermont; Midwest: Illinois, Indiana, Iowa, Kansas, Michigan, Minnesota, Missouri, Nebraska, North Dakota, Ohio, South Dakota, and Wisconsin; South: Alabama, Arkansas, Delaware, District of Columbia, Florida, Georgia, Kentucky, Louisiana, Maryland, Mississippi, North Carolina, Oklahoma, South Carolina, Tennessee, Texas, Virginia, and West Virginia, and West: Alaska, Arizona, California, Colorado, Hawaii, Idaho, Montana, Nevada, New Mexico, Oregon, Utah, Washington, and Wyoming. §§ Includes Medicare, Worker's Compensation, self-pay, no charge/charity, and other. ¶¶ NAMCS data available for 2006–2010 only, with 21,526,910 mean annual visits. *** Includes general/family practice, internal medicine, and pediatrics. ††† Visits with symptoms and signs that should prompt a chlamydia test, including mucopurulent cervicitis, pelvic inflammatory disease, abnormal vaginal discharge, dyspareunia, postcoital bleeding, abnormal vaginal bleeding, or dysuria. §§§ Includes birth control, sterilization, and abortion counseling, examination, or provision. ¶¶¶ NAMCS data available only for 2007–2010, with 21,656,080 mean annual visits. **** Sum of subgroups might not match overall total due to inclusion of different years of data or rounding. |
||||||
FIGURE. Percentage of sexually active females aged 16–20 years with commercial and Medicaid insurance and percentage of Title X family planning clinic users aged 15–19 years who were tested for chlamydia — United States, 1999–2010
* Source: Healthcare Effectiveness Data and Information Set, United States, 1999–2010. Available at http://www.ncqa.org/ReportCards/HealthPlans/StateofHealthCareQuality.aspx.
† Source: Title X data set, United States, 2005–2010. Available at http://www.hhs.gov/opa/title-x-family-planning/research-and-data/fp-annual-reports.
Alternate Text: The figure shows the percentage of sexually active females aged 16-20 years with commercial and Medicaid insurance and percentage of Title X family planning clinic users aged 15-19 years who were tested for chlamydia in the United States during 1999-2010. Data for females with commercial and Medicaid insurance are drawn from the Healthcare Effectiveness Data and Information Set, United States, 1999-2010. Data for Title X family planning clinic users are drawn from the Title X data set, United States, 2005-2010. Percentages for all groups increased during the study period, with percentages for females with commercial and Medicaid insurance being consistently higher than that for family planning clinic users.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All MMWR HTML versions of articles are electronic conversions from typeset documents.
This conversion might result in character translation or format errors in the HTML version.
Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr)
and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S.
Government Printing Office (GPO), Washington, DC 20402-9371;
telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to
mmwrq@cdc.gov.


