FluView Summary ending on March 2, 2024
Updated March 8, 2024

Seasonal influenza activity remains elevated nationally with increases in some parts of the country.
Viruses
All data are preliminary and may change as more reports are received.
Directional arrows indicate changes between the current week and the previous week. Additional information on the arrows can be found at the bottom of this page.
A description of the CDC influenza surveillance system, including methodology and detailed descriptions of each data component is available on the surveillance methods page.
Additional information on the current and previous influenza seasons for each surveillance component are available on FluView Interactive.
- Seasonal influenza activity remains elevated nationally with increases in some parts of the country.
- Nationally, percent positivity for influenza remained stable compared to last week. Trends in percent positivity for influenza A and B varied by region.
- Nationally, outpatient respiratory illness declined slightly but remains above baseline.1 Regions 8 and 10 are below their respective baselines during Week 9 for the first time since early and mid-November, respectively, while the remaining HHS regions remain above their respective baselines.
- The number of weekly flu hospital admissions remained stable compared to last week. After five weeks of sharp decline between late December and early February, the number of weekly flu hospital admissions has been trending downward slightly since mid-February.
- During Week 9, of the 615 viruses reported by public health laboratories, 437 (71.1%) were influenza A and 178 (28.9%) were influenza B. Of the 286 influenza A viruses subtyped during Week 9, 164 (57.3%) were influenza A(H1N1) and 122 (42.7%) were A(H3N2).
- Ten influenza-associated pediatric deaths were reported during Week 9, bringing the 2023-2024 season total to 103 pediatric deaths.
- CDC estimates that there have been at least 28 million illnesses, 310,000 hospitalizations, and 20,000 deaths from flu so far this season.
- CDC recommends that everyone 6 months and older get an annual flu vaccine as long as influenza viruses are spreading.2 Vaccination can still provide benefit this season.
- There also are prescription flu antiviral drugs that can treat flu illness; those should be started as early as possible and are especially important for higher risk patients.3
- Flu viruses are among several viruses contributing to respiratory disease activity. CDC is providing updated, integrated information about COVID-19, flu, and RSV activity on a weekly basis.
U.S. Virologic Surveillance
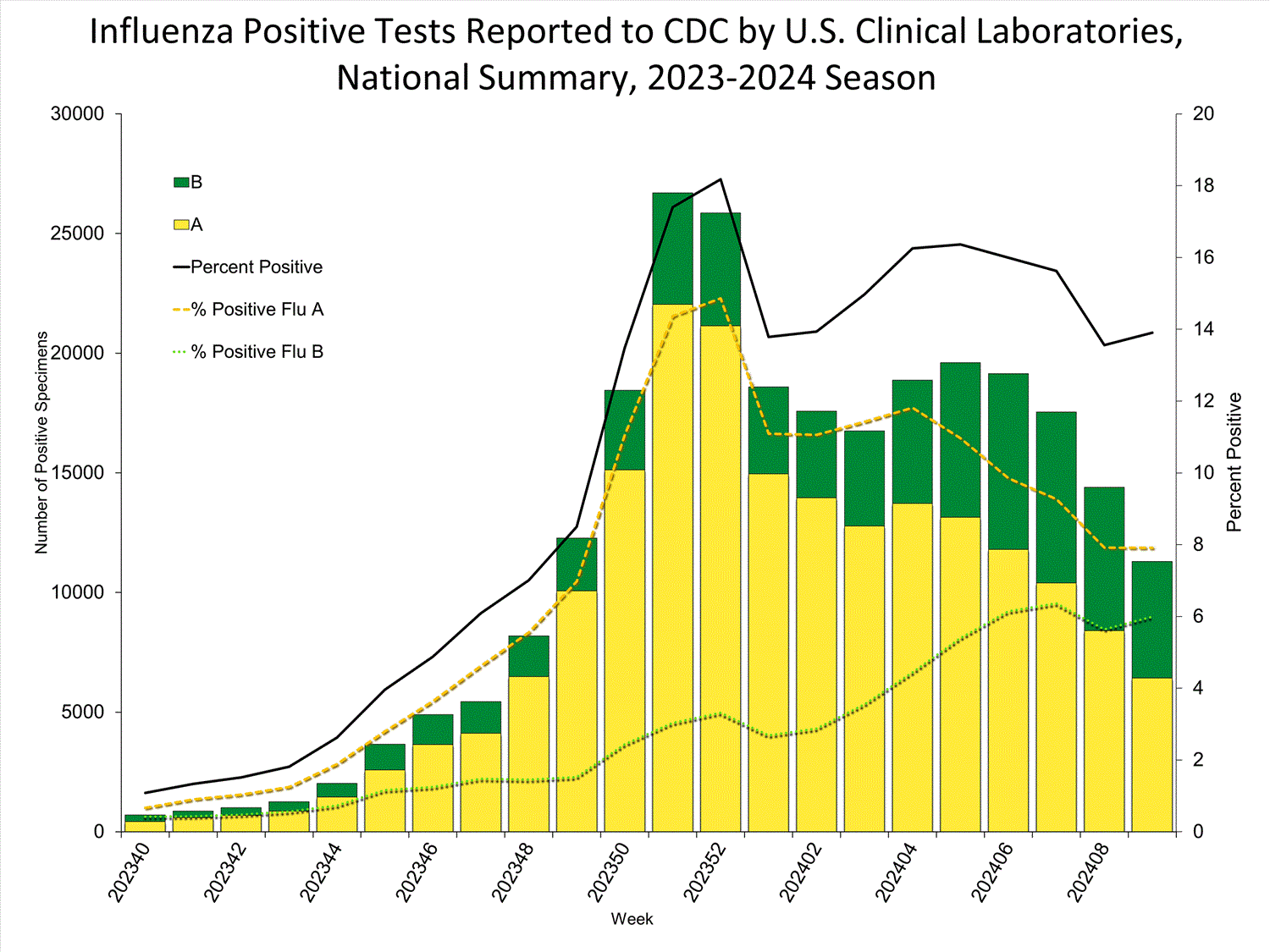
Nationally, the percentage of respiratory specimens testing positive for influenza in clinical laboratories remained stable (change of <0.5 percentage points) compared to the previous week, but trends varied by region. Region 5 reported an increase; regions 1, 2, 4, 6, and 8 reported a decrease; and regions 3, 7, 9, and 10 remained stable during Week 9 compared to Week 8. The regions with the highest percent positivity were regions 7 (27.9%), 5 (19.8%), and 3 (17.4%). Since Week 40, influenza A(H1N1)pdm09 has been the predominant virus circulating in all regions. However, the distribution of circulating viruses varies by region. For regional and state level data and age group distribution, please visit FluView Interactive. Viruses known to be associated with recent receipt of live attenuated influenza vaccine (LAIV) or found upon further testing to be a vaccine virus are not included, as they are not circulating influenza viruses.
Clinical Laboratories
The results of tests performed by clinical laboratories nationwide are summarized below. Data from clinical laboratories (the percentage of specimens tested that are positive for influenza virus) are used to monitor whether influenza activity is increasing or decreasing.
| Week 9 |
Data Cumulative since October 1, 2023 (Week 40) |
|
|---|---|---|
| No. of specimens tested | 81,237 | 2,346,992 |
| No. of positive specimens (%) | 11,294 (13.9%) | 265,084 (11.3%) |
| Positive specimens by type | ||
| Influenza A | 6,428 (56.9%) | 194,842 (73.5%) |
| Influenza B | 4,866 (43.1%) | 70,232 (26.5%) |
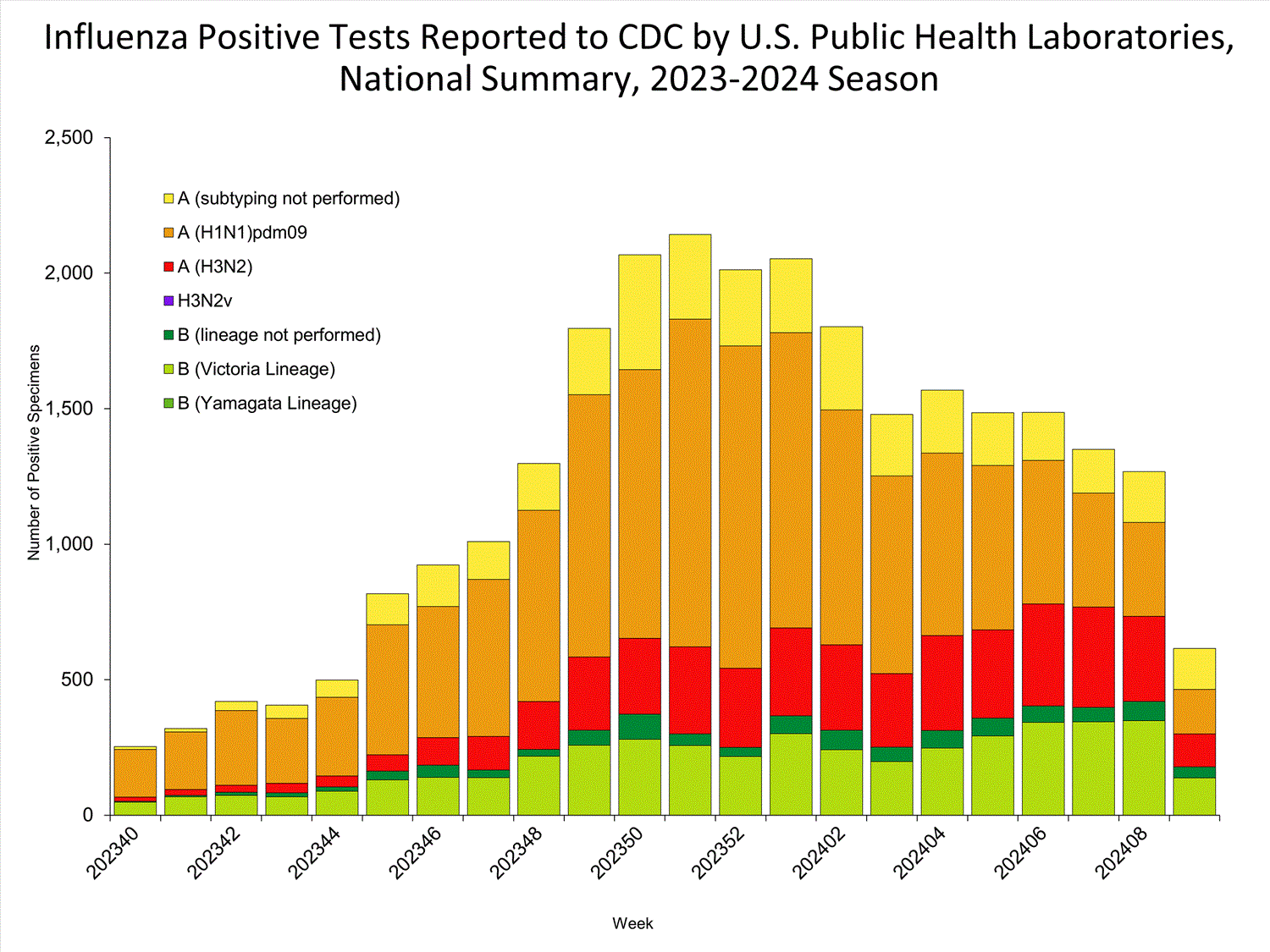
Public Health Laboratories
The results of tests performed by public health laboratories nationwide are summarized below. Data from public health laboratories are used to monitor the proportion of circulating influenza viruses that belong to each influenza subtype/lineage.
| Week 9 |
Data Cumulative since October 1, 2023 (Week 40) |
|
|---|---|---|
| No. of specimens tested | 2,541 | 83,981 |
| No. of positive specimens | 615 | 27,069 |
| Positive specimens by type/subtype | ||
| Influenza A | 437 (71.1%) | 21,684 (80.1%) |
| Subtyping Performed | 286 (65.4%) | 17,762 (81.9%) |
| (H1N1)pdm09 | 164 (57.3%) | 13,221 (74.4%) |
| H3N2 | 122 (42.7%) | 4,541 (25.6%) |
| H3N2v | 0 (0.0%) | 0 (0.0%) |
| Subtyping not performed | 151 (34.6%) | 3,922 (18.1%) |
| Influenza B | 178 (28.9%) | 5,385 (19.9%) |
| Lineage testing performed | 138 (77.5%) | 4,441 (82.5%) |
| Yamagata lineage | 0 (0.0%) | 0 (0.0%) |
| Victoria lineage | 138 (100%) | 4,441 (100%) |
| Lineage not performed | 40 (22.5%) | 944 (17.5%) |
Additional virologic surveillance information for current and past seasons:
Surveillance Methods | FluView Interactive: National, Regional, and State Data or Age Data
Influenza Virus Characterization
CDC performs genetic and antigenic characterization of U.S. viruses submitted from state and local public health laboratories according to the Right Size Roadmap submission guidance. These data are used to compare how similar the currently circulating influenza viruses are to the reference viruses representing viruses contained in the current influenza vaccines. The data are also used to monitor evolutionary changes that continually occur in influenza viruses circulating in humans. CDC also tests susceptibility of circulating influenza viruses to antiviral medications including the neuraminidase inhibitors (oseltamivir, zanamivir, and peramivir) and the PA endonuclease inhibitor baloxavir.
CDC has genetically characterized 2,227 influenza viruses collected since October 1, 2023.
| Virus Subtype or Lineage | Genetic Characterization | ||||
|---|---|---|---|---|---|
| Total No. of Subtype/Lineage Tested |
HA Clade |
Number (% of subtype/lineage tested) |
HA Subclade |
Number (% of subtype/lineage tested) |
|
| A/H1 | 998 | ||||
| 6B.1A.5a | 998 (100%) | 2a | 254 (25.5%) | ||
| 2a.1 | 744 (74.5%) | ||||
| A/H3 | 639 | ||||
| 3C.2a1b.2a | 639 (100%) | 2a.1b | 1 (0.2%) | ||
| 2a.3a | 1 (0.2%) | ||||
| 2a.3a.1 | 636 (99.5%) | ||||
| 2b | 1 (0.2%) | ||||
| B/Victoria | 590 | ||||
| V1A | 590 (100%) | 3a.2 | 590 (100%) | ||
| B/Yamagata | 0 | ||||
| Y3 | 0 | Y3 | 0 (0%) | ||
CDC antigenically characterizes influenza viruses by hemagglutination inhibition (HI) (H1N1pdm09, H3N2, B/Victoria, and B/Yamagata viruses) or neutralization-based HINT (H3N2 viruses) using antisera that ferrets make after being infected with reference viruses representing the 2023-2024 Northern Hemisphere recommended cell or recombinant-based vaccine viruses. Antigenic differences between viruses are determined by comparing how well the antibodies made against the vaccine reference viruses recognize the circulating viruses that have been grown in cell culture. Ferret antisera are useful because antibodies raised against a particular virus can often recognize small changes in the surface proteins of other viruses. In HI assays, viruses with similar antigenic properties have antibody titer differences of less than or equal to 4-fold when compared to the reference (vaccine) virus. In HINT, viruses with similar antigenic properties have antibody neutralization titer differences of less than or equal to 8-fold. Viruses selected for antigenic characterization are a subset representing the genetic changes in the surface proteins seen in genetically characterized viruses.
Influenza A Viruses
- A (H1N1)pdm09: 172 A(H1N1)pdm09 viruses were antigenically characterized by HI, and all were well-recognized (reacting at titers that were within 4-fold of the homologous virus titer) by ferret antisera to cell-grown A/Wisconsin/67/2022-like reference viruses representing the A(H1N1)pdm09 component for the cell- and recombinant-based influenza vaccines.
- A (H3N2): 161 A(H3N2) viruses were antigenically characterized by HI or HINT, and 159 (99%) were well-recognized (reacting at titers that were within 4-fold of the homologous virus titer in HI or reacting at titers that were less than or equal to 8-fold of the homologous virus in HINT) by ferret antisera to cell-grown A/Darwin/6/2021-like reference viruses representing the A(H3N2) component for the cell- and recombinant-based influenza vaccines.
Influenza B Viruses
- B/Victoria: One hundred influenza B/Victoria-lineage virus were antigenically characterized by HI, and all were well-recognized (reacting at titers that were within 4-fold of the homologous virus titer) by ferret antisera to cell-grown B/Austria/1359417/2021-like reference viruses representing the B/Victoria component for the cell- and recombinant-based influenza vaccines.
- B/Yamagata: No influenza B/Yamagata-lineage viruses were available for antigenic characterization.
2024-2025 Influenza Season – U.S. Influenza Vaccine Composition:
The World Health Organization (WHO) has recommended the Northern Hemisphere 2024-2025 influenza vaccine composition, and the Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee (VRBPAC) subsequently made the influenza vaccine composition recommendation for the United States. Both agencies recommend that influenza vaccines contain the following:
Egg-based vaccines
-
- an A/Victoria/4897/2022 (H1N1)pdm09-like virus;
- an A/Thailand/8/2022 (H3N2)-like virus; and
- a B/Austria/1359417/2021 (B/Victoria lineage)-like virus.
Cell- or recombinant-based vaccines
-
- an A/Wisconsin/67/2022 (H1N1)pdm09-like virus;
- an A/Massachusetts/18/2022 (H3N2)-like virus; and
- a B/Austria/1359417/2021 (B/Victoria lineage)-like virus.
The Committee recommended that all 2024-2025 U.S. flu vaccines be three-component (trivalent) vaccines and include an influenza A(H1N1), an A(H3N2) and a B/Victoria-lineage vaccine virus. Because influenza B/Yamagata viruses, which are included in current four-component (quadrivalent) influenza vaccines, are no longer actively circulating, their inclusion in flu vaccines is no longer warranted. Further information on vaccine composition for the 2024-2025 influenza season can be found on the CDC Spotlight.
Assessment of Virus Susceptibility to Antiviral Medications
CDC assesses susceptibility of influenza viruses to the antiviral medications including the neuraminidase inhibitors (oseltamivir, zanamivir, and peramivir) and the PA endonuclease inhibitor baloxavir using next generation sequence analysis supplemented by laboratory assays. Information about antiviral susceptibility test methods can be found at U.S. Influenza Surveillance: Purpose and Methods | CDC.
Viruses collected in the U.S. since October 01, 2023, were tested for antiviral susceptibility as follows:
| Antiviral Medication | Total Viruses | A/H1 | A/H3 | B/Victoria | ||
|---|---|---|---|---|---|---|
| Neuraminidase Inhibitors | Oseltamivir | Viruses Tested | 2,230 | 998 | 640 | 592 |
| Reduced Inhibition | 1 (0.4%) | 1 (0.1%) | 0 (0.0%) | 0 (0.0%) | ||
| Highly Reduced Inhibition | 1 (0.4%) | 1 (0.1%) | 0 (0.0%) | 0 (0.0%) | ||
| Peramivir | Viruses Tested | 2,230 | 998 | 640 | 592 | |
| Reduced Inhibition | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | ||
| Highly Reduced Inhibition | 1 (0.4%) | 1 (0.1%) | 0 (0.0%) | 0 (0.0%) | ||
| Zanamivir | Viruses Tested | 2,230 | 998 | 640 | 592 | |
| Reduced Inhibition | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | ||
| Highly Reduced Inhibition | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | ||
| PA Cap-Dependent Endonuclease Inhibitor | Baloxavir | Viruses Tested | 2,167 | 974 | 623 | 570 |
| Decreased Susceptibility | 1 (0.5%) | 0 (0.0%) | 1 (0.2%) | 0 (0.0%) | ||
One A(H1N1)pdm09 virus had NA-H275Y amino acid substitution and showed highly reduced inhibition by oseltamivir and peramivir. One (H1N1)pdm09 virus had NA-I223V and NA-S247N amino acid substitutions and showed reduced inhibition by oseltamivir.
One A(H3N2) virus had PA-I38T amino acid substitution and showed reduced susceptibility to baloxavir.
High levels of resistance to the adamantanes (amantadine and rimantadine) persist among influenza A(H1N1)pdm09 and influenza A(H3N2) viruses (the adamantanes are not effective against influenza B viruses). Therefore, use of these antivirals for treatment and prevention of influenza A virus infection is not recommended and data from adamantane resistance testing are not presented.
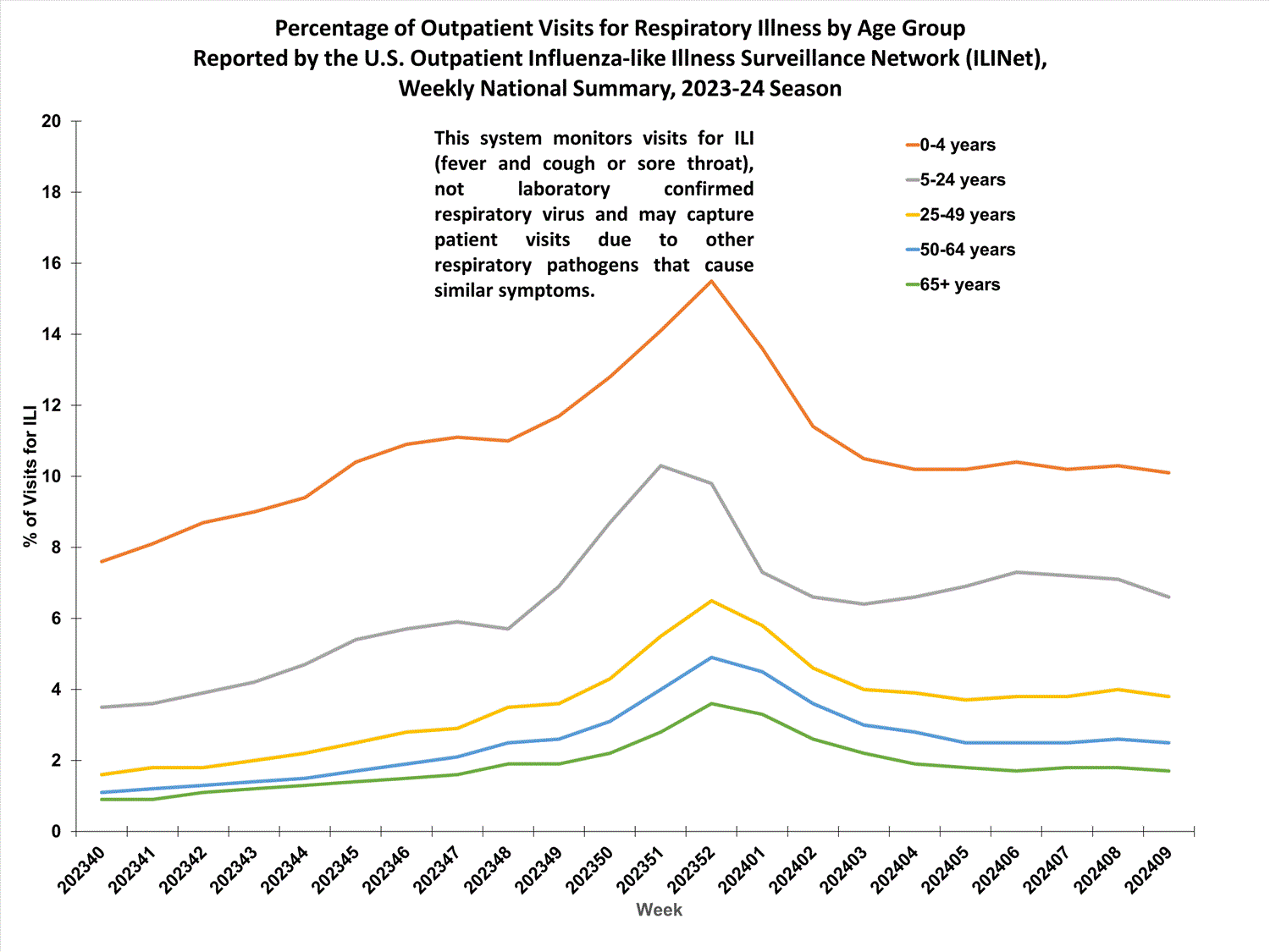
Outpatient Respiratory Illness Surveillance
The U.S. Outpatient Influenza-like Illness Surveillance Network (ILINet) monitors outpatient visits for respiratory illness referred to as influenza-like illness [ILI (fever plus cough or sore throat)], not laboratory-confirmed influenza and will therefore capture respiratory illness visits due to infection with pathogens that can present with similar symptoms, including influenza viruses, SARS-CoV-2, and RSV. It is important to evaluate syndromic surveillance data, including that from ILINet, in the context of other sources of surveillance data to obtain a more complete and accurate picture of influenza, SARS-CoV-2, and other respiratory virus activity. CDC is providing integrated information about COVID-19, influenza, and RSV activity on a website that is updated weekly. Information about other respiratory virus activity can be found on CDC’s National Respiratory and Enteric Virus Surveillance System (NREVSS) website.
Outpatient Respiratory Illness Visits
Nationwide, during Week 9, 4.1% of patient visits reported through ILINet were due to respiratory illness that included fever plus a cough or sore throat, also referred to as ILI. This has decreased (change of > 0.1 percentage points) compared to Week 8. The percentage of visits for ILI remained stable in regions 2, 3, 5, and 7, and decreased in regions 1, 4, 6, 8, 9, and 10 in Week 9 compared to Week 8. Regions 8 and 10 are below their region-specific baselines in Week 9 for the first time since early and mid-November respectively, while all other regions remain above their region-specific baselines. Multiple respiratory viruses are co-circulating, and the relative contribution of influenza virus infection to ILI varies by location.
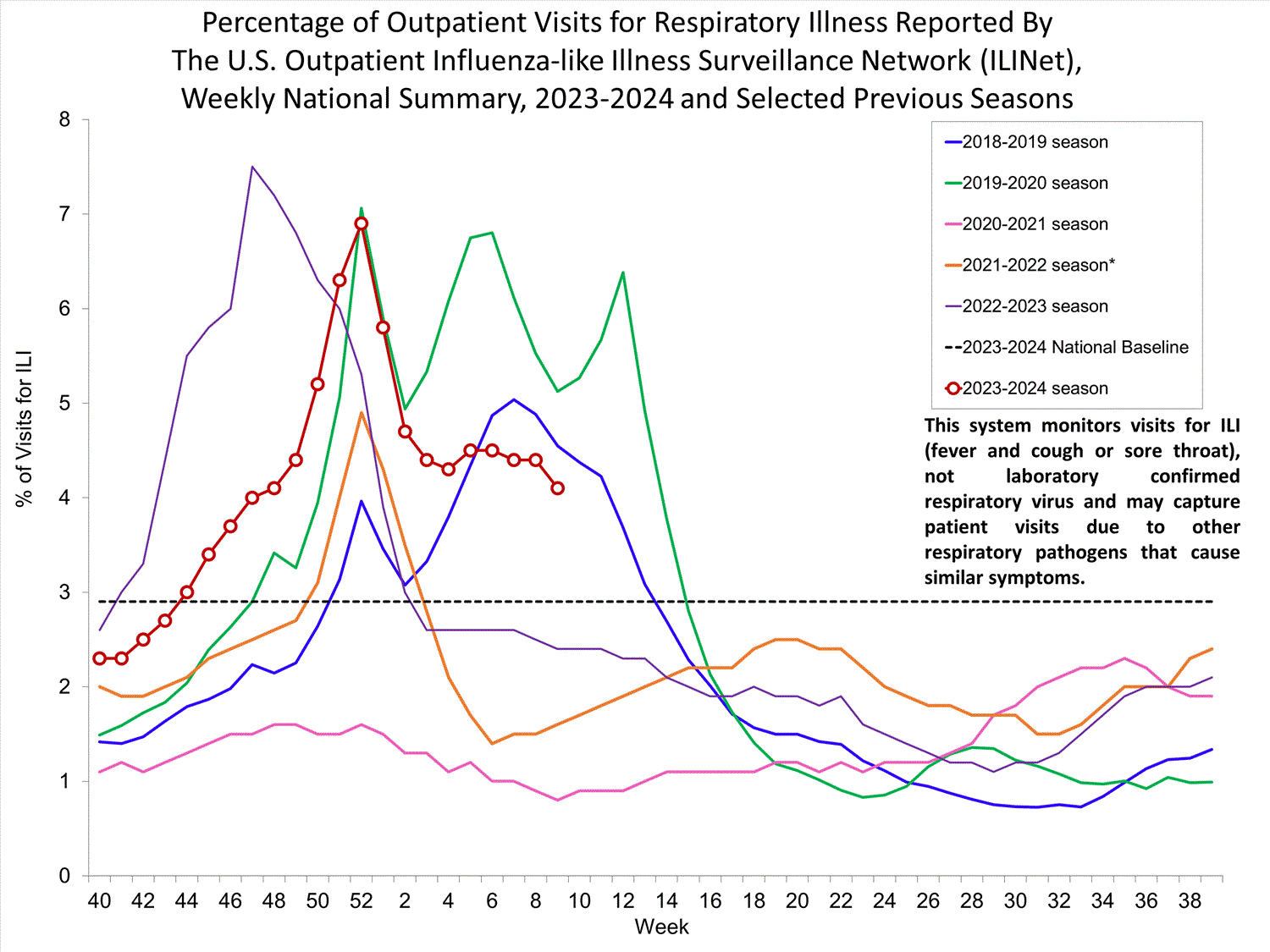
* Effective October 3, 2021 (week 40), the ILI definition (fever plus cough or sore throat) no longer includes “without a known cause other than influenza.”
Outpatient Respiratory Illness Visits by Age Group
About 70% of ILINet participants provide both the number of patient visits for respiratory illness and the total number of patient visits for the week broken out by age group. Data from this subset of providers are used to calculate the percentages of patient visits for respiratory illness by age group.
The percentage of visits for respiratory illness reported in ILINet decreased in the 0-4 years, 5-24 years, and 25-49 years age groups and remained stable in the 50-64 years and 65+ years age groups during Week 9 compared to Week 8.
Outpatient Respiratory Illness Activity Map
Data collected in ILINet are used to produce a measure of ILI activity* by state/jurisdiction and Core Based Statistical Areas (CBSA).
| Activity Level | Number of Jurisdictions | Number of CBSAs | ||
|---|---|---|---|---|
| Week 9 (Week ending Mar. 2, 2024) |
Week 8 (Week ending Feb. 24, 2024) |
Week 9 (Week ending Mar. 2, 2024) |
Week 8 (Week ending Feb. 24, 2024) |
|
| Very High | 5 | 5 | 16 | 16 |
| High | 15 | 21 | 92 | 109 |
| Moderate | 18 | 13 | 99 | 120 |
| Low | 9 | 7 | 201 | 209 |
| Minimal | 8 | 8 | 296 | 251 |
| Insufficient Data | 0 | 1 | 225 | 224 |
*Data collected in ILINet may disproportionally represent certain populations within a jurisdiction or CBSA, and therefore, may not accurately depict the full picture of influenza activity for the entire jurisdiction or CBSA. Differences in the data presented here by CDC and independently by some health departments likely represent differing levels of data completeness with data presented by the health department likely being the more complete.
Additional information about medically attended visits for ILI for current and past seasons:
Surveillance Methods | FluView Interactive: National, Regional, and State Data or ILI Activity Map
Hospitalization Surveillance
FluSurv-NET
The Influenza Hospitalization Surveillance Network (FluSurv-NET) conducts population-based surveillance for laboratory-confirmed influenza-related hospitalizations in select counties in 14 states and represents approximately 9% of the U.S. population. FluSurv-NET hospitalization data are preliminary. As data are received each week, prior case counts and rates are updated accordingly.
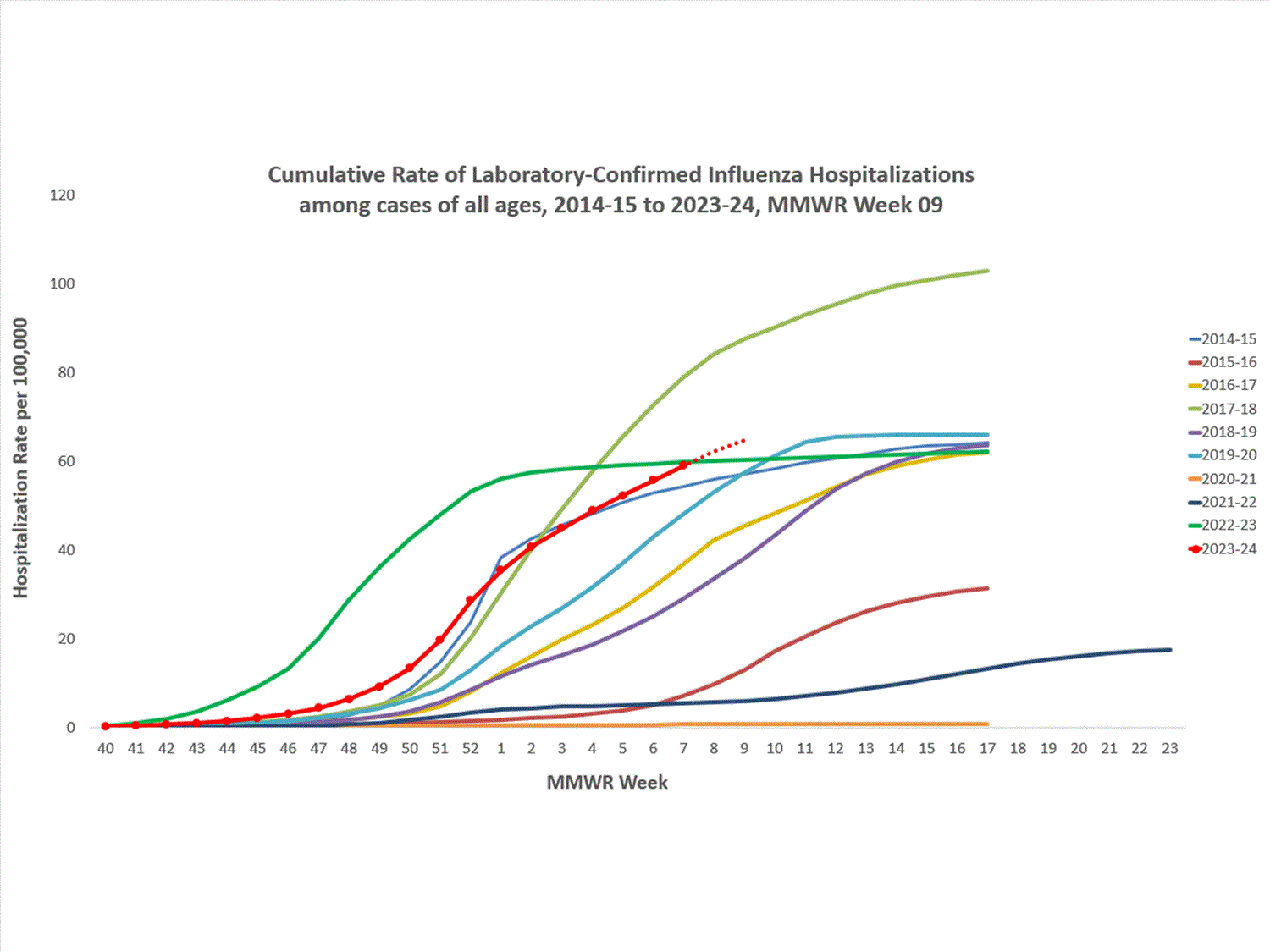
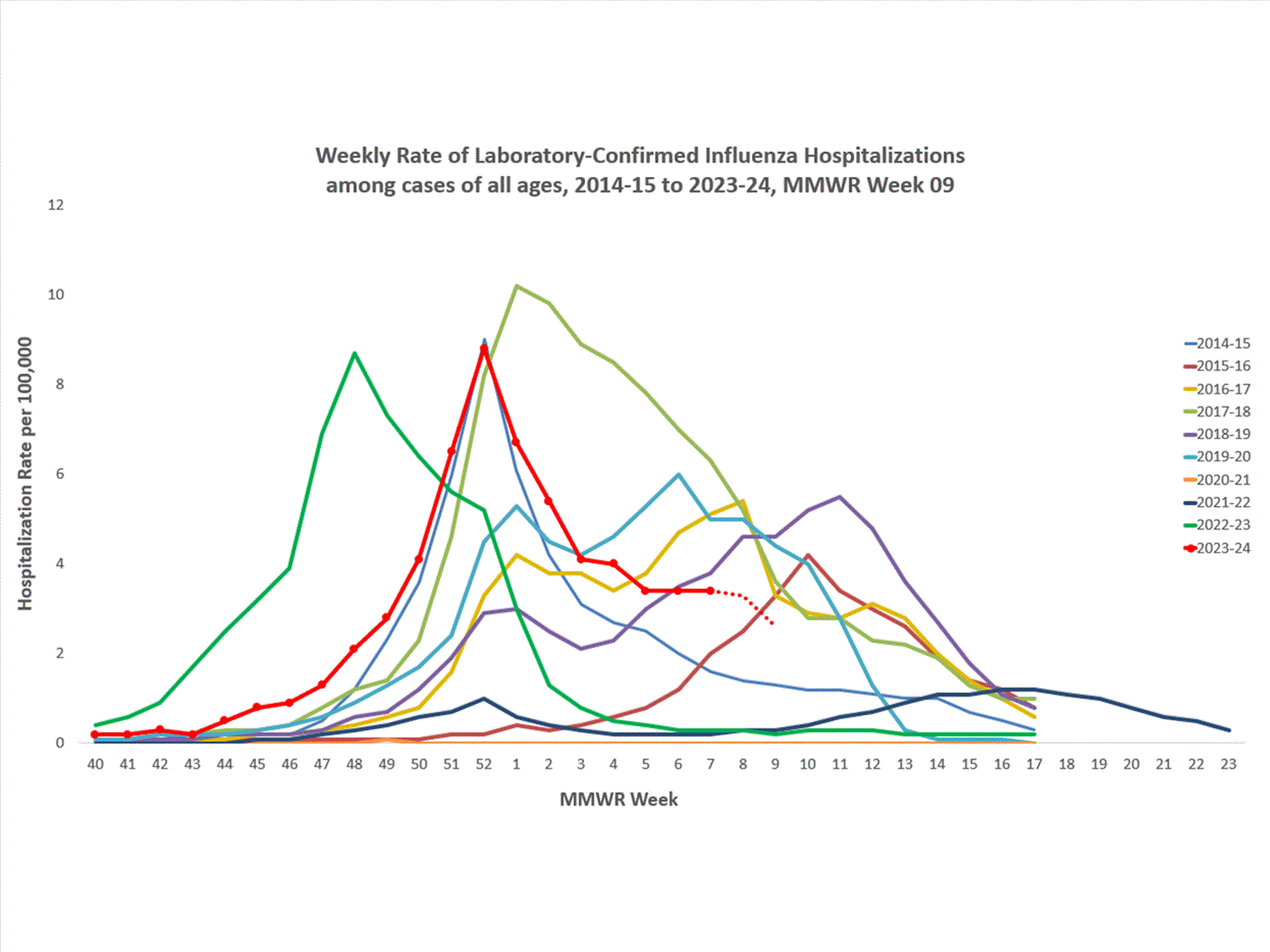
A total of 19,819 laboratory-confirmed influenza-associated hospitalizations were reported by FluSurv-NET sites between October 1, 2023, and March 2, 2024. The weekly hospitalization rate observed in Week 9 was 2.6 per 100,000 population. The weekly hospitalization rate observed during Week 52 is the third highest peak weekly rate observed during all seasons going back to 2010-2011, following the 2014-2015 and 2017-2018 seasons. The overall cumulative hospitalization rate was 64.8 per 100,000 population. This cumulative hospitalization rate is the second highest cumulative hospitalization rate when compared against previous end-of-season rates for Week 9, and it is the second highest cumulative in-season hospitalization rate observed in Week 9, following the 2017-2018 season (86.3). Cumulative in-season hospitalization rates observed in Week 9 from 2010-2011 through 2022-2023 ranged from 0.7 to 60.0.
When examining rates by age, the highest cumulative hospitalization rate per 100,000 population was among adults aged 65 years and older (175.8), followed by adults aged 50-64 years (78.5) and children aged 0-4 years (67.6). When examining age-adjusted rates by race and ethnicity, the highest rate of hospitalization per 100,000 population was among non-Hispanic Black persons (124.1), followed by non-Hispanic American Indian or Alaska Native persons (90.1), Hispanic persons (61.2), non-Hispanic White persons (49.3), and non-Hispanic Asian/Pacific Islander persons (34.9).
Among 19,819 hospitalizations, 17,163 (86.6%) were associated with influenza A virus, 2,504 (12.6%) with influenza B virus, 39 (0.2%) with influenza A virus and influenza B virus co-infection, and 112 (0.6%) with influenza virus for which the type was not determined. Among those with influenza A subtype information, 2,901 (73.1%) were A(H1N1) pdm09 and 1,066 (26.9%) were A(H3N2).
Among 2,168 hospitalized adults with information on underlying medical conditions, 95.6% had at least one reported underlying medical condition, the most commonly reported were hypertension, cardiovascular disease, obesity, and metabolic disease. Among 1,140 hospitalized women of childbearing age (15-49 years) with information on pregnancy status, 23.3% were pregnant. Among 633 hospitalized children with information on underlying medical conditions, 69.3% had at least one reported underlying medical condition; the most commonly reported was asthma, followed by obesity and neurologic disease.
In this figure, cumulative rates for all seasons prior to the 2023-2024 season reflect end-of-season rates. For the 2023-2024 season, rates for recent hospitals admissions are subject to reporting delays. As hospitalization data are reviewed each week, prior case counts and rates are updated accordingly.
In this figure, weekly rates for all seasons prior to the 2023-24 season reflect end-of-season rates. For the 2023-24 season, rates for recent hospital admissions are subject to reporting delays and are shown as a dashed line for the current season. As hospitalization data are received each week, prior case counts and rates are updated accordingly.
Additional FluSurv-NET hospitalization surveillance information for current and past seasons and additional age groups:
Surveillance Methods |FluView Interactive: Rates by Age, Sex, and Race/Ethnicity or Data on Patient Characteristics | RESP-NET Interactive
National Healthcare Safety Network (NHSN) Hospitalization Surveillance
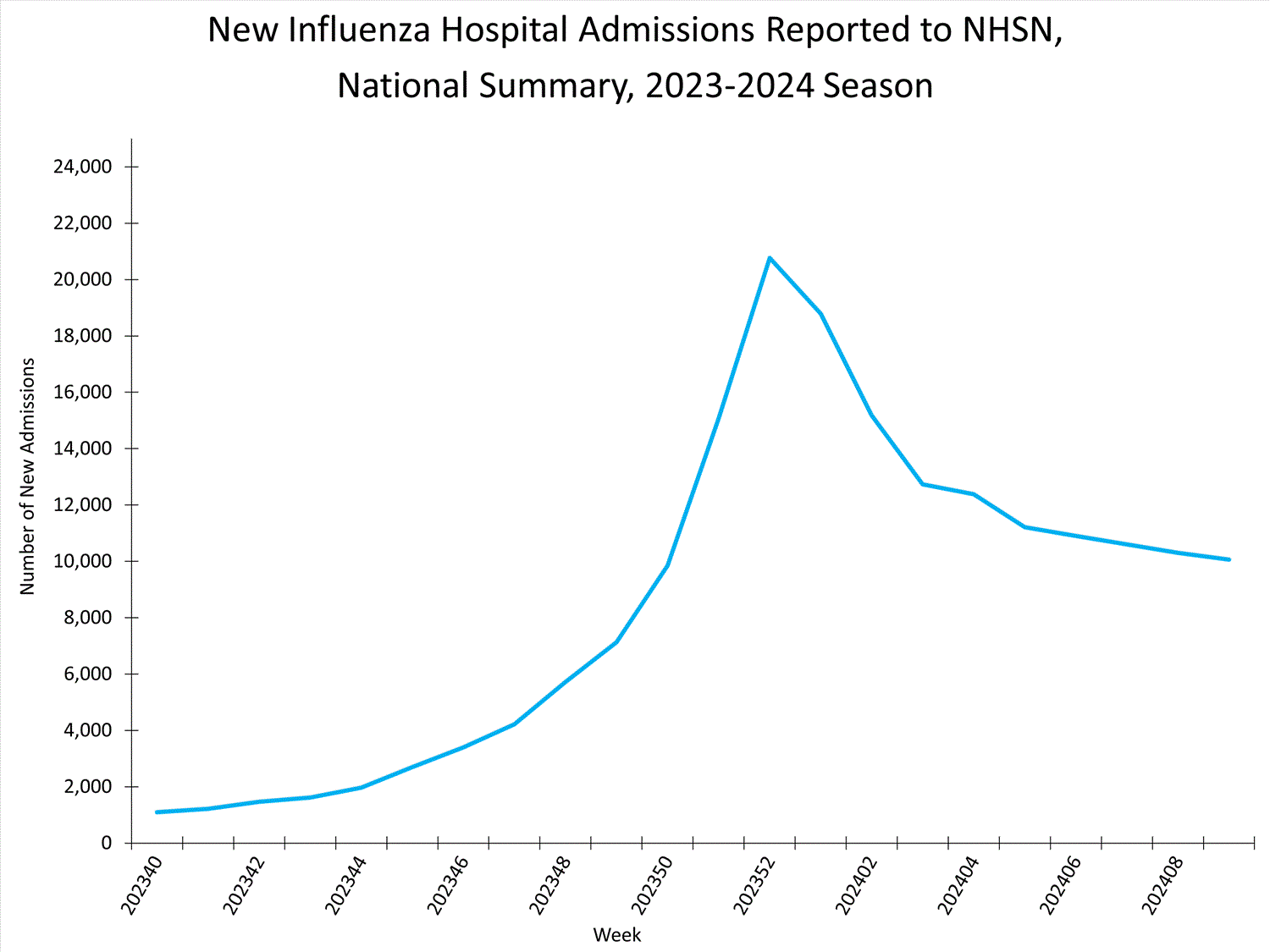
Hospitals report to NHSN the weekly number of patients admitted with laboratory-confirmed influenza. During Week 9, 10,060 patients with laboratory-confirmed influenza were admitted to a hospital. Nationally, the number of patients admitted to a hospital with laboratory-confirmed influenza for Week 9 remained stable (change of <5%) compared to Week 8, and after five weeks of sharp decline between late December and early February, the number of weekly flu hospital admissions has been trending downward slightly since mid-February. The number of hospitalizations increased in regions 1 and 3, remained stable in regions 4, 5, and 7, and decreased in regions 2, 6, 8, 9, and 10 this week compared to Week 8.
Additional NHSN Hospitalization Surveillance information:
Surveillance Methods | Additional Data | FluView Interactive
Mortality Surveillance
National Center for Health Statistics (NCHS) Mortality Surveillance
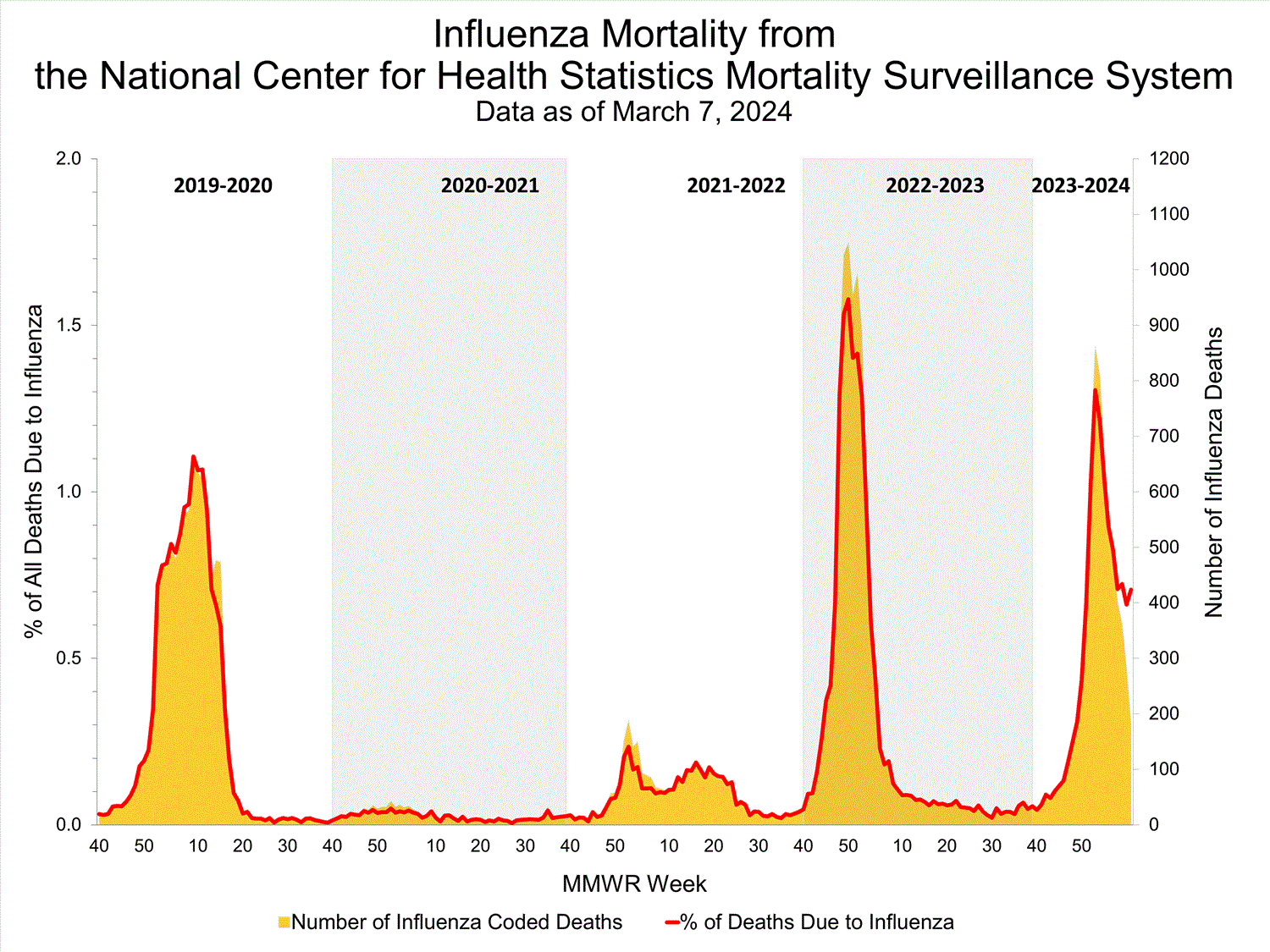
Based on NCHS mortality surveillance data available on March 7, 2024, 0.7% of the deaths that occurred during the week ending March 2, 2024 (Week 9), were due to influenza. This percentage remained stable (< 0.1 percentage point change) compared to Week 8. The data presented are preliminary and may change as more data are received and processed.
Additional pneumonia, influenza and COVID-19 mortality surveillance information for current and past seasons:
Surveillance Methods | FluView Interactive
Influenza-Associated Pediatric Mortality
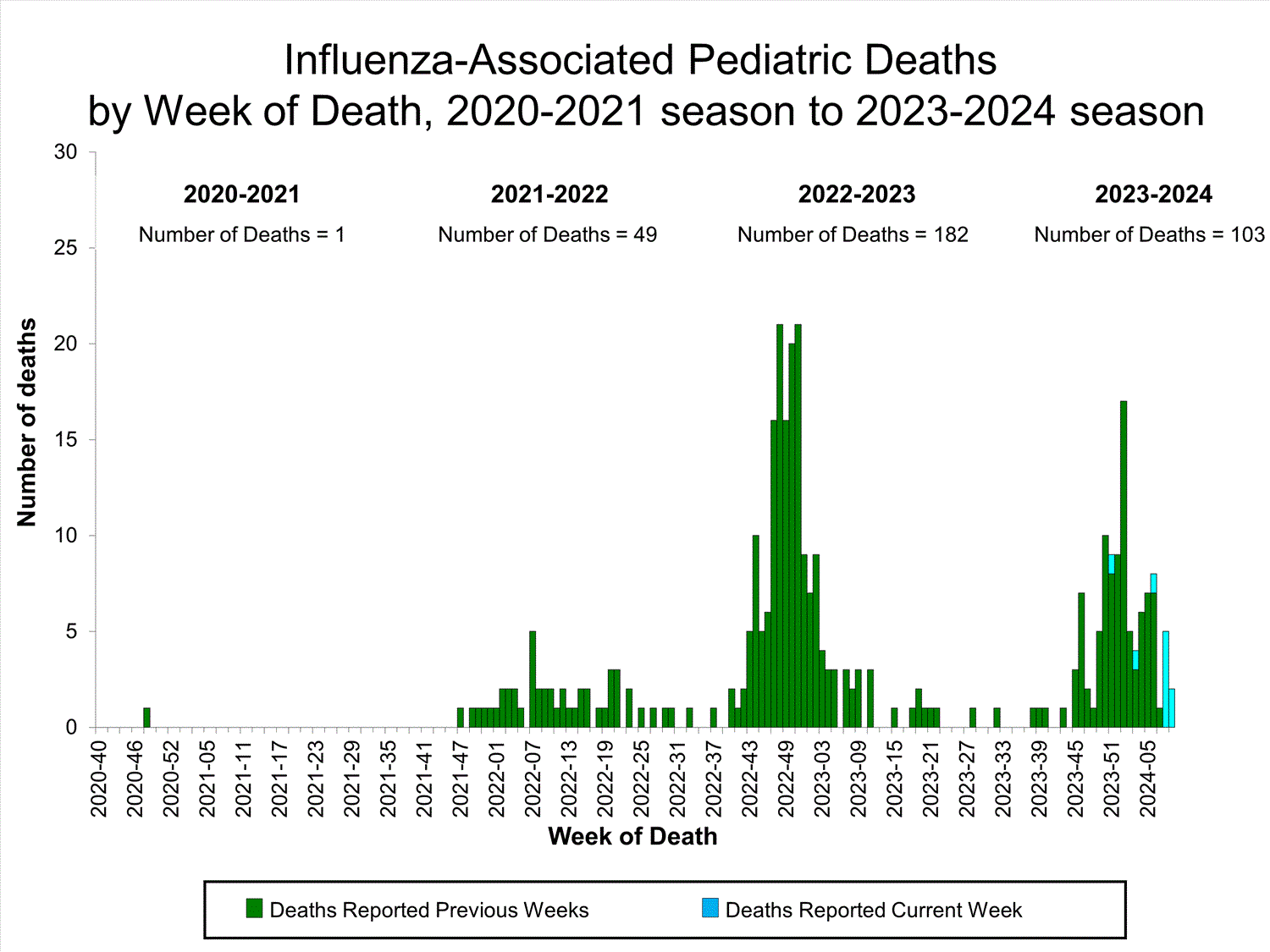
Ten influenza-associated pediatric deaths occurring during the 2023-2024 season were reported to CDC during Week 9. The deaths occurred during Week 51 of 2023 (the week ending December 23, 2023) and between weeks 3 and 9 of 2024 (the weeks ending January 20, 2024, and March 2, 2024). Six deaths were associated with influenza A viruses. Two of the influenza A viruses had subtyping performed; they were A(H1N1) and A(H3) viruses. Four deaths were associated with influenza B viruses, one of which was determined be a B/Victoria lineage-virus.
A total of 103 influenza-associated pediatric deaths occurring during the 2023-2024 season have been reported to CDC.
Additional pediatric mortality surveillance information for current and past seasons:
Surveillance Methods | FluView Interactive
Trend Indicators
Increasing: 
Decreasing: 
Stable: 
Indicators Status by System
Clinical Labs: Up or down arrows indicate a change of greater than or equal to 0.5 percentage points in the percent of specimens positive for influenza compared to the previous week.
Outpatient Respiratory Illness (ILINet): Up or down arrows indicate a change of greater than 0.1 percentage points in the percent of visits due to respiratory illness (ILI) compared to the previous week.
NHSN Hospitalizations: Up or down arrows indicate change of greater than or equal to 5% of the number of patients admitted with laboratory-confirmed influenza compared to the previous week.
NCHS Mortality: Up or down arrows indicate change of greater than 0.1 percentage points of the percent of deaths due to influenza compared to the previous week.
Reference Footnotes
1U.S. Influenza Surveillance: Purpose and Methods (2023 Oct). Centers for Disease Control and Prevention. https://www.cdc.gov/flu/weekly/overview.htm#ILINet.
2Grohskopf LA, Blanton LH, Ferdinands JM, Chung JR, Broder KR, Talbot HK. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices — United States, 2023–24 Influenza Season. MMWR Recomm Rep 2023;72(No. RR-2):1–25. DOI: http://dx.doi.org/10.15585/mmwr.rr7202a1
3Influenza Antiviral Medications: Summary for Clinicians (2023 Sept). Centers for Disease Control and Prevention. https://www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm.
Additional National and International Influenza Surveillance Information
FluView Interactive: FluView includes enhanced web-based interactive applications that can provide dynamic visuals of the influenza data collected and analyzed by CDC. These FluView Interactive applications allow people to create customized, visual interpretations of influenza data, as well as make comparisons across flu seasons, regions, age groups and a variety of other demographics.
National Institute for Occupational Safety and Health: Monthly surveillance data on the prevalence of health-related workplace absenteeism among full-time workers in the United States are available from NIOSH.
U.S. State and local influenza surveillance: Select a jurisdiction below to access the latest local influenza information.
World Health Organization:
Additional influenza surveillance information from participating WHO member nations is available through
FluNet and the Global Epidemiology Reports.
WHO Collaborating Centers for Influenza:
Australia, China, Japan, the United Kingdom, and the United States (CDC in Atlanta, Georgia)
Europe:
The most up-to-date influenza information from Europe is available from WHO/Europe and the European Centre for Disease Prevention and Control.
Public Health Agency of Canada:
The most up-to-date influenza information from Canada is available in Canada’s weekly FluWatch report.
Public Health England:
The most up-to-date influenza information from the United Kingdom is available from Public Health England.
Any links provided to non-Federal organizations are provided solely as a service to our users. These links do not constitute an endorsement of these organizations or their programs by CDC or the Federal Government, and none should be inferred. CDC is not responsible for the content of the individual organization web pages found at these links.
A description of the CDC influenza surveillance system, including methodology and detailed descriptions of each data component is available on the surveillance methods page.