October 2022
October 18, 2022, 3:00–4:30 PM ET: Regular October eSHARE webinar: “Updates on COVID-19 surveillance cadence, information about new features for the annual NNDSS data tables on CDC WONDER, and CDC enhancements to case surveillance.”
October 25, 2022, 3:00-4:00 PM ET: eSHARE special session: “What you need to know about NNDSS and the Mpox Response.” CDC will provide a specific date and time for the special session soon.
December 1, 2022: Deadline to:
- sign off on final 2021 reconciliation data and
- send NNDSS final aggregate 2021 COVID-19 case counts.
Coming soon: New interactive data tool for NNDSS annual data on CDC WONDER
As part of CDC’s efforts to modernize public health data systems, CDC’s Wide-ranging Online Data for Epidemiologic Research (WONDER) and the National Notifiable Diseases Surveillance System (NNDSS) are excited to announce the upcoming release of a new interactive tool for users of NNDSS annual data. A key data source for public health, the NNDSS annual tables provide information on cases of selected infectious national notifiable diseases and conditions sent to CDC by U.S. states and territories.

The new interactive tool will:
- Allow users of NNDSS annual data to create data visualizations customized to their needs.
- Offer more flexibility to focus their analyses and understand trends and patterns, such as comparing NNDSS data across years, specific populations, diseases and conditions, and geographic areas.
- Enhance user experience compared to the current, fixed format annual data tables.
- Make NNDSS data more transparent.
With the release of these new interactive features, CDC is not adding any new data elements to NNDSS or the ability to stratify existing NNDSS data elements in ways not previously available. CDC expects to release the new interactive tool in late 2022 and will notify everyone when it is available.
If you have questions about these upcoming features, contact cwus@cdc.gov.
CDC to require the 2020 RVCT for 2023 tuberculosis case data
Beginning in 2023, jurisdictions will need to use the 2020 Report of Verified Case of Tuberculosis (RVCT) when sending their TB data to CDC.
All TB cases counted in 2023 must be submitted using the 2020 RVCT.
Jurisdictions’ 2022 TB cases do not need to use the 2020 RVCT, even if the cases are transmitted to CDC in 2023. For example, a jurisdiction sending 2022 TB cases in January 2023 will still be able to use the previous RVCT for those cases.
Key changes in the 2020 RVCT:
- New items include new anti-TB drugs, new drug-susceptibility testing methods, census tract, date of symptom onset, and additional risk factors, including smoking, ever experiencing homelessness, or residing in a correctional facility.
- Repeating group blocks allow flexibility in the number of responses for certain variables, including laboratory and imaging results, TB medications, epidemiologic linkages, and additional TB risk factors.
Questions that have been removed include:
- type of laboratory or outpatient health provider
- initial visa type
- whether the person was under custody of Immigration and Customs Enforcement.
Jurisdictions that would like an alternative to using the 2020 RVCT can submit their TB case data to CDC using direct data entry into the National TB Surveillance System – Case Reports (NTSS-CR). If you have questions about this option, please contact the CDC Division of Tuberculosis Elimination Support Helpdesk at DTBESupport@cdc.gov.
If you have any questions about the 2020 RVCT, please contact DTBESupport@cdc.gov.
Need help during 2021 annual reconciliation? NNDSS surveillance officers are here to help guide you through the process!
- Keaton Hughes (qwy4@cdc.gov): AL, AK, CA, HI, KS, ME, MA, MN, MO, NE, NC, ND, NYC, OR, PR, SC, VT, VA
- Diana Onweh (onw1@cdc.gov): AR, CO, CT, GA, ID, IA, IL, IN, LA, MD, MI, MS, NH, NV, PA, TN, TX, WA, WY
- Alan Schley (aso7@cdc.gov): AZ, DE, FL, KY, MT, NJ, NM, NY, OH, OK, RI, SD, UT, DC, WV, WI, territories
An eSHARE special session, “What you need to know about NNDSS and the Mpox Response,” will be held October 25, 2022, 3:00-4:00 PM ET.
The regular October eSHARE—which will provide updates on COVID-19 surveillance cadence, information about new features for the annual NNDSS data tables on CDC WONDER, and CDC enhancements to case surveillance—was held October 18, 2022, 3:00—4:30 PM ET.
To join an eSHARE webinar, please see your calendar invitation or contact the CDC Electronic Data Exchange mailbox at edx@cdc.gov for login information with the subject line “eSHARE invitation.”
COVID-19 Message Mapping Guide
Version 1.2 of the COVID-19 message mapping guide has now posted to the NNDSS website, which adds the data element Lineage (INV1405).
The PHIN VADS COVID-19 Case Notification View version 13 is now available for COVID-19 MMG versions 1.0, 1.1, and 1.2. The changes include:
- 37 additional values to Risk Factor (COVID-19) (now v.2),
- 13 additional values to Treatment Type (COVID-19) to include medication treatment options, including Paxlovid (now v.2),
- 12 additional values (6 new US vaccines and 6 non-US) to Vaccine Type (NND) (now v.11), and
- one non-COVID additional value for Manufacturers of vaccines (MVX) (now v.14).
Additionally, version 1.2 of the “COVID Lite” test case scenarios worksheet (TCSW) and associated test scenarios have been posted to the NNDSS website. This updated version incorporates both Lineage (INV1405) and First Specimen Collection Date (95366-1) in addition to the GenV2 and vaccine data elements.
If you are interested in joining a cohort or onboarding an MMG, please email the CDC Electronic Data Exchange mailbox at edx@cdc.gov.
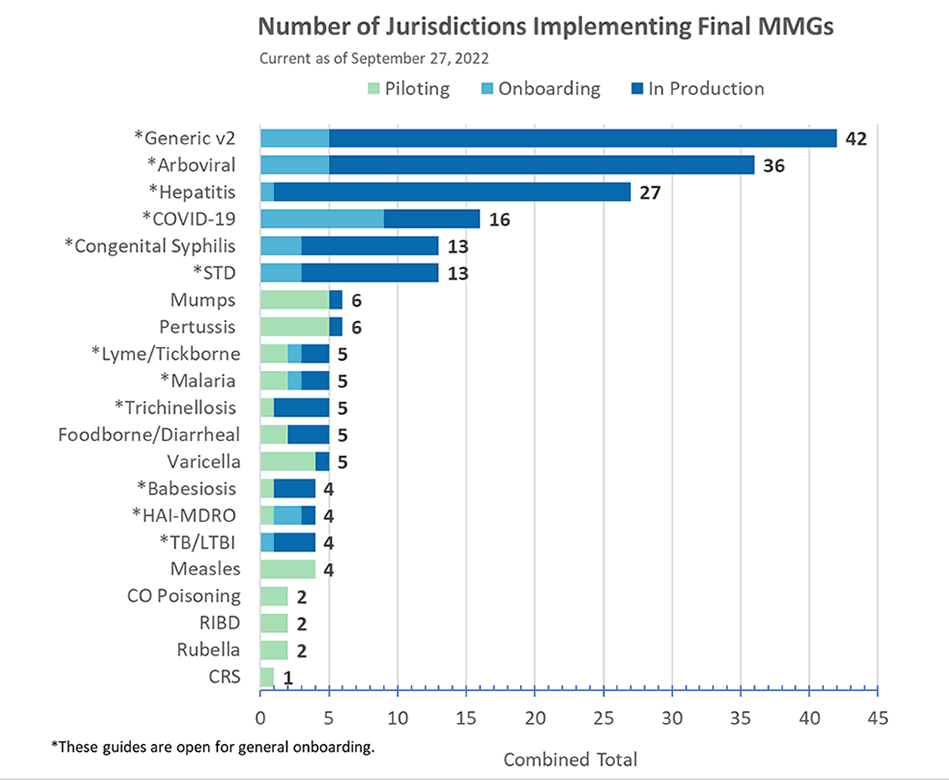
Congratulations to the following jurisdiction(s) in production as of September 27!
- Florida for Babesia and Trichinellosis message mapping guides
- Oregon for STD version 1.2 and CS version 1.1 message mapping Guides
In Case You Missed It: Actions for Jurisdictions During 2021 Reconciliation
On August 16, 2021, the CDC NNDSS team kicked off the process for reconciliation of 2021 annual data. CDC uses the annual reconciliation process to confirm that information in jurisdictions’ databases matches information in the CDC NNDSS database before finalizing data for each year.
Here are the key actions your jurisdiction will need to take to complete the 2021 reconciliation process:
- Review CDC NNDSS data and resolve discrepancies using the reconciliation tables provided by your NNDSS Surveillance Officer along with the line list and case summary in the Message Validation, Processing, and Provisioning System (MVPS) portal.
- Send case notifications to CDC to correct differences. You may request a new set of reconciliation tables at any time.
- Notify your NNDSS Surveillance Officer to “lock” your 2021 NNDSS data when you have finished reviewing and updating your data.
- Review and provide final sign-off from your State or Territorial Epidemiologist after you receive updated tables of your locked data.
The deadline for State and Territorial Epidemiologist approval of the final 2021 data is December 1, 2022.
Reminder: Process for 2021 Final NNDSS COVID-19 Data
The 2021 final NNDSS COVID-19 data will not be reconciled; however, CDC will collect final 2021 NNDSS aggregate COVID-19 case counts.
CDC will provide jurisdictions with an Excel spreadsheet containing instructions and information about the aggregate data needed for the 2021 NNDSS tables.
- Jurisdictions will submit their final aggregate case counts in an Epi Info™ web form. The Excel spreadsheet will provide jurisdictions with a preview of the data that will need to be entered into the Epi Info™ web form.
- State and Territorial Epidemiologists will “sign off” on the aggregate 2021 COVID-19 case counts within the Epi Info™ web form.
- CDC will use aggregate case counts in the 2021 NNDSS annual tables.
The tables will include confirmed, probable, and total cases of COVID-19 among U.S. residents, with stratifications by jurisdiction of residence, sex, age group, a combined race and ethnicity field, and month (based on Morbidity and Mortality Weekly Report weeks).
Jurisdictions should plan to send their final aggregate 2021 COVID-19 case counts to CDC by December 1, 2022. This is the same deadline for submitting final 2021 data for other NNDSS conditions.
Learning Resources for 2021 NNDSS Reconciliation
CDC has made additional guidance documents with more detailed steps in the NNDSS reconciliation process available within the reconciliation section of the MVPS portal.
CDC provided training on the 2021 reconciliation process during an eSHARE training webinar on August 16, 2022. Visit the eSHARE archives to access the slides and recordings for past eSHARE webinars.