Phase 1 (of 3): Pre-onboarding
‹View Table of Contents

This page gives jurisdiction health departments submitting data to the National Notifiable Diseases Surveillance System (NNDSS) a closer look at how to complete the message mapping guide (MMG) pre-onboarding phase.
During the pre-onboarding phase, a jurisdiction notifies CDC of plans to implement an MMG and moves through stages to prepare for the onboarding phase. Technical assistance needs may be accessed during this phase.
(The arboviral MMG pre-onboarding and onboarding process is slightly different. Learn more about how to implement arboviral case notifications.)
How to Start Pre-onboarding
The best way to start pre-onboarding is to notify CDC of your interest in implementing an MMG by sending an email to edx@cdc.gov with the subject “[Jurisdiction name]: [MMG name] Pre-Onboarding.”
Other ways your jurisdiction can let CDC know that you are interested in implementing an MMG include:
- Epidemiology and Laboratory Capacity for Prevention and Control of Emerging Infectious Diseases (ELC) calls
- An NNDSS ELC Health Information Systems update in REDCap
- Learning of an upcoming cohort. Cohorts are formed when 3 or more jurisdictions express interest in—and capacity to implement—an MMG. If a cohort cannot be formed, individual technical assistance is available. Jurisdictions often learn of new cohorts and express interest to CDC in joining one by attending an eSHARE training webinar.
CDC may also contact your jurisdiction to ask if you are interested in onboarding a specific MMG.
Request Technical Assistance
Technical assistance is available at any stage of the onboarding process. If you request technical assistance, the Association of Public Health Laboratories (APHL) will contact you to schedule a readiness assessment. APHL will walk you through a series of questions to determine if the jurisdiction has the resources and data ready to begin working on the MMG.
For jurisdictions not using technical assistance or joining a cohort for pre-onboarding, CDC will send the onboarding package to APHL for review during pre-onboarding.
Stages of Pre-onboarding
There are five stages of pre-onboarding:
These stages are designed to identify and resolve common issues early in the implementation process. Your jurisdiction can move through these stages with or without technical assistance.
Stage 1: Readiness Assessment
- Identify which MMGs you intend to implement. Find currently available message mapping guides on the NNDSS Technical Resource Center under the HL7 Message Mapping Guides & Standards page. You can also find supporting documents for implementation such as the PHIN Messaging Specification for Case Notification (PHIN Spec).
- Conduct a readiness assessment of your technical infrastructure, test environment, transport, expertise, and resources by using the readiness checklist and reviewing the infrastructure questions.
- Identify your team:
- project lead/champion,
- lead for integrated surveillance system,
- person responsible for gap analysis,
- person responsible for creating electronic messages,
- person responsible for configuring message transport, and
- person responsible for data administration of surveillance system for conditions covered by selected MMG.
- Gather technical documentation, such as case investigation forms, electronic laboratory reporting message examples, data extracts, and technical architecture diagrams and workflows.
Stage 2: Gap Analysis
During Gap Analysis, compare MMG data elements against the information contained in your jurisdiction’s surveillance systems, including:
- existing data elements,
- data elements derived from script and logic creation, and
- elements created or added to the jurisdiction’s surveillance system.
Jurisdictions must send the data elements noted in the MMG as required for the HL7 case notification message. If a required data element is not present in your jurisdiction’s surveillance system, you will need to add it before onboarding.
The NNDSS Implementation Spreadsheet can help facilitate gap analysis. Annotate which data elements within your disease surveillance system (DSS) already exist, which you can derive, and which you will need to build. Also consider the feasibility or timeline for the development work needed. The figure below demonstrates how the NNDSS Implementation Spreadsheet merges information from the MMGs, the PHIN Spec, and PHIN Vocabulary Access and Distribution System (PHIN VADS).
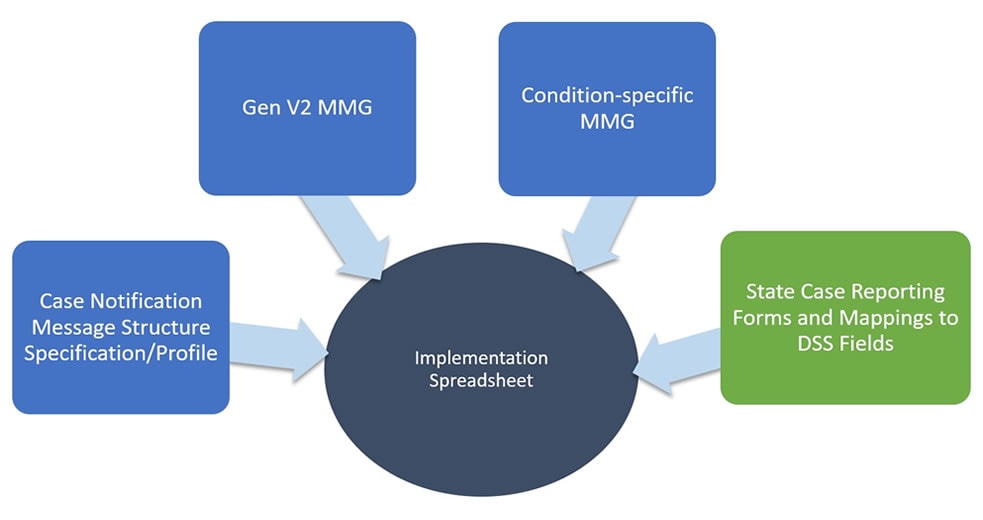
Sources for the MMG Implementation Spreadsheets
The CDC program reviews and provides feedback on the implementation spreadsheet. This allows the jurisdiction to make necessary adjustments before starting work on system updates.
Before making system updates, jurisdictions should send an initial copy of the implementation spreadsheet to edx@cdc.gov. The email subject line should be “[Jurisdiction name]: [MMG name] IS For Review.”
Stage 3: Message Creation
Make system updates and changes after the CDC program reviews and provides feedback on the implementation spreadsheet.
After you update your jurisdiction’s surveillance system, create a data extract of all MMG elements indicated in the MMG implementation spreadsheet.
Enter test messages associated with the test case scenario worksheet. Indicate any differences from what CDC provides in the scenario.
Stage 4: Route Creation for Transport
Develop a transport route for your jurisdiction to send HL7 messages to CDC. The transport route will need to generate and send case notification messages in accordance with the MMG and PHIN Messaging Specification for Case Notification.
CDC and its partners have developed a template Rhapsody channel based on the MMG specifications. Jurisdictions can use this channel as a baseline to develop a route that will transform and translate data from your jurisdiction’s surveillance system to create valid HL7 messages.
To discuss available transport options, send an email to edx@cdc.gov with the subject “[Jurisdiction name]: [MMG name] – Transport Options.”
Stage 5: Message Validation
Using the CDC-provided MMG test package, enter the test messages into your jurisdiction’s surveillance system using the test scenarios accompanying the MMG. The test messages provide data for each field when data are logically possible for the condition. The expected number of test messages differs by MMG.
Validate generated HL7 messages using the Message Evaluation and Testing Service (METS). METS provides feedback on message structure and content. Messages are not transported to CDC during validation.
During this iterative process, make all needed changes to your jurisdiction’s surveillance system and data extract until all test messages successfully pass METS without errors.
Investigate warnings. If you cannot resolve a warning—such as a warning for a missing, non-required data field that the jurisdiction does not collect—document the warning and inform CDC so that everyone knows what to expect during the validation process. The number of test messages your jurisdiction must develop varies by MMG; please refer to the MMG test case scenario worksheet.
Get Access to MVPS Portal
In preparation for onboarding, obtain CDC Secure Access Management Services (SAMS) Level 2 access for all users who will need access to the Message Validation, Processing, and Provisioning System (MVPS) portal.
Your jurisdiction data managers can request access for you by sending an email with the subject line of “MVPS New User” to the EDX mailbox (edx@cdc.gov). The email should include the new user’s name(s), email address(es), and desired MVPS role(s).
You can also request access by sending an email directly to the EDX mailbox. To streamline the process, include an approval email from your jurisdiction data manager with your request. If you don’t provide an approval email, CDC will request confirmation from your jurisdiction data managers. If you don’t know who your jurisdiction data managers are, send an email with subject line of “MVPS Access Help” to the EDX mailbox at edx@cdc.gov.
Transition to Onboarding Phase
If your jurisdiction did not receive technical assistance or participate in a cohort, CDC will send the onboarding package to APHL for review before starting the onboarding phase. Once you have addressed all feedback, APHL will help you submit the final onboarding package to CDC to begin the onboarding phase.
If your jurisdiction did receive technical assistance or participate in a cohort, APHL will help you submit the final onboarding package to CDC to begin the onboarding phase.
Submit Onboarding Package
Request to start onboarding by sending an email to edx@cdc.gov with the information listed below. Include the name of your jurisdiction and the MMG you want to onboard in the subject line of the email: “[Jurisdiction name] – NNDSS Onboarding Package for [MMG].” In the email, confirm that your jurisdiction has:
- requested CDC Secure Access Management Services (SAMS) Level 2 access and MVPS portal access for all necessary users,
- identified your jurisdiction data manager and your backup jurisdiction data manager roles in MVPS,
- validated that test messages have passed the Message Evaluation and Testing Service (METS) with no errors and limited warnings, and
- confirmed the transport mechanism is in place for both test and production environments.
If applicable, include confirmation that your jurisdiction’s PHIN Messaging System certificates are up to date. Additionally, include or attach in the email:
- list of contact information and roles for the jurisdiction’s key stakeholders;
- list of all NNDSS diseases that are state reportable and will be sent for the MMG being onboarded;
- completed NNDSS Implementation Spreadsheet, located under artifacts on the specific MMG page; and
- a completed NNDSS Test Case Scenario Worksheet with jurisdiction-specific data to show mappings and which data elements are in the surveillance system.
CDC will review your final onboarding package and assess CDC capacity available for onboarding your jurisdiction.
Onboarding Kick-off Call
If there is capacity to onboard your jurisdiction for the MMG, then CDC will schedule an onboarding kick-off meeting with you to:
- outline the onboarding process,
- decide on appropriate project timelines,
- resolve outstanding questions or issues with documentation,
- confirm the transportation method for sending data to CDC, and
- ensure the correct people have access to the Message Validation, Provisioning, and Processing System (MVPS) portal.
After you’ve held an onboarding kick-off call with CDC, you’re ready to start stage 1 of onboarding. CDC considers jurisdictions to be officially in the onboarding phase after the kick-off meeting.