August 2022
December 1, 2022: Deadline to:
- sign off on final 2021 reconciliation data and
- send NNDSS final aggregate 2021 COVID-19 case counts.
NNDSS Begins Reconciliation of 2021 Annual Data
CDC expects to begin the process of reconciling 2021 annual NNDSS data in August 2022. CDC uses the annual reconciliation process to confirm that information in jurisdictions’ databases matches information in the CDC NNDSS database before finalizing data for each year.
Jurisdictions should ensure that necessary staff have access to the Message Validation, Processing, and Provisioning System (MVPS) portal. Jurisdiction Data Managers can request access for their jurisdictions’ staff by emailing the CDC Electronic Data Exchange mailbox at edx@cdc.gov with subject: “New MVPS User Access.”
What to Expect

The process of reconciling 2021 data will be very similar to the process used to reconcile 2020 data.
To start the process, a CDC NNDSS surveillance officer will email each jurisdiction a reconciliation packet.
Once a jurisdiction receives their reconciliation packet, an NNDSS surveillance officer will be available to help the jurisdiction through the rest of the reconciliation process. The reconciliation packet will include:
- a letter with an overview of the changes to the reconciliation process for 2021 NNDSS data,
- a production calendar with a timeline of key dates in the process,
- a week-ending calendar for 2021,
- an abbreviated event code list for 2021, and
- a copy of the jurisdiction’s reconciliation tables and instructions for reviewing the reconciliation tables.
During reconciliation, jurisdictions may use the reconciliation tables provided by their NNDSS surveillance officer along with the line list and case summary in the MVPS portal to review CDC NNDSS data and resolve discrepancies.
Jurisdictions should send case notifications to CDC to correct any differences and may request a new set of reconciliation tables at any time.
Jurisdictions should notify their NNDSS surveillance officer to “lock” their data when they have finished reviewing and updating their 2021 NNDSS data. After locking a jurisdiction’s data, the NNDSS surveillance officer will send updated reconciliation tables for final review and sign-off by the State or Territorial Epidemiologist. The deadline for State and Territorial Epidemiologist approval of the final 2021 data is December 1, 2022.
Process for 2021 Final NNDSS COVID-19 Data
The 2021 final NNDSS COVID-19 data will not be reconciled; however, CDC will collect final 2021 NNDSS aggregate COVID-19 case counts.
CDC will provide jurisdictions with an Excel spreadsheet containing instructions and information about the aggregate data needed for the 2021 NNDSS tables.
- Jurisdictions will submit their final aggregate case counts in an Epi Info™ web form. The Excel spreadsheet will provide jurisdictions with a preview of the data that will need to be entered into the Epi Info™ web form.
- State and Territorial Epidemiologists will “sign off” on the aggregate 2021 COVID-19 case counts within the Epi Info™ web form.
- CDC will use aggregate case counts in the 2021 NNDSS annual tables.
The tables will include confirmed, probable, and total cases of COVID-19 among U.S. residents, with stratifications by jurisdiction of residence, sex, age group, a combined race and ethnicity field, and month (based on Morbidity and Mortality Weekly Report weeks).
Jurisdictions should plan to send their final aggregate 2021 COVID-19 case counts to CDC by December 1, 2022. This is the same deadline for submitting final 2021 data for other NNDSS conditions.
Learning Resources for NNDSS Reconciliation
CDC has made additional guidance documents with more detailed steps in the NNDSS reconciliation process available within the reconciliation section of the MVPS portal.
CDC provided training on the 2021 reconciliation process during an eSHARE training webinar on August 16, 2022. Visit the eSHARE archives to access the slides and recordings for past eSHARE webinars. CDC typically posts eSHARE slides and recordings a few days after each webinar.
Need help during 2021 annual reconciliation? NNDSS surveillance officers are here to help guide you through the process!
- Keaton Hughes (qwy4@cdc.gov): AL, AK, CA, HI, KS, ME, MA, MN, MO, NE, NC, ND, NYC, OR, PR, SC, VT, VA
- Diana Onweh (onw1@cdc.gov): AR, CO, CT, GA, ID, IA, IL, IN, LA, MD, MI, MS, NH, NV, PA, TN, TX, WA, WY
- Alan Schley (aso7@cdc.gov): AZ, DE, FL, KY, MT, NJ, NM, NY, OH, OK, RI, SD, UT, DC, WV, WI, territories
Update on Mpox Case Notification to CDC
CDC is making updates to the mpox case surveillance process. We are transitioning to implementation of the short case report form (sCRF) for reporting mpox cases to CDC and have updated the CDC mpox webpage for health departments with the sCRF and data dictionary.
CDC has retired the processes for reporting cases via the call center and via the original case report form (oCRF) in REDCap. However, CDC will be able to accept bulk upload files with the oCRF data elements for a short time, until jurisdictions can fully transition to the sCRF. All jurisdictions should begin to transition to the sCRF at this time.
For official case counts, the current method for submitting data to CDC is to send data directly to DCIPHER via direct entry, bulk upload, or an application programming interface (API).
CDC is using DCIPHER to compile mpox response data from multiple sources, including all case data from the call center and case report forms. Cases received in DCIPHER prior to the 2pm data cut off will be included in CDC case counts for that day and reflected on the CDC website. Please refer to the updated case reporting page on the website for more details.
How to Send Mpox Case Data to DCIPHER
- Enter the necessary sCRF data directly in the CDC DCIPHER platform or
- Enter the necessary data directly into an existing jurisdictional case surveillance system configured for mpox reporting. Then upload the reporting data to the CDC DCIPHER platform using a CSV file format or using an API.
To ensure timely reporting, initial reports can be submitted to CDC daily with only two minimum required data elements: Local Record ID and Case jurisdiction of residence. CDC asks that these initial records be updated with additional data as soon as possible as case investigations continue.
How to Prepare to Submit Mpox Initial Case Notifications Through NNDSS
Soon, CDC will implement NNDSS reporting for mpox case data. We are currently building the process to incorporate the data from NNDSS into DCIPHER. After onboarding to NNDSS, core data elements will be sent through NNDSS. Disease-specific data elements in the sCRF should still be sent directly to DCIPHER.
To initiate validation for your NNDSS transmission, please email the CDC Electronic Data Exchange mailbox at edx@cdc.gov with the subject “MPX validation.” The validation will confirm your:
- NNDSS submission and
- Case count after we merge data from NNDSS with other cases you have reported to the CDC Mpox Response.
You will be notified when your NNDSS data are being used in DCIPHER for official case counts.
In Case You Missed It: CDC Memorandum to State and Territorial Epidemiologists about Mpox
Position statement 22-ID-10, titled Public Health Reporting and National Notification for Mpox Virus Infection, was approved by the Council of State and Territorial Epidemiologists (CSTE) on June 23, 2022.
Jurisdictions should use the CSTE case definition described in the position statement for cases reported on or before August 1, 2022. Jurisdictions should not retroactively change the classification of cases reported prior to August 1, 2022, based on the new case definition.
CDC will post the mpox national surveillance case definition on the NNDSS case definitions webpage as soon as possible. Until that time, refer to the case definition in the CSTE position statement.
CSTE recommends that all states and territories make mpox virus infection reportable in their jurisdiction. Jurisdictions are encouraged to send all 2022 mpox cases to NNDSS, including all cases from previous months that were reported through the CDC Mpox Call Center, REDCap, DCIPHER direct entry, bulk CSV upload, or API. CDC’s NNDSS has assigned event code 11801 to mpox virus infection.
The CSTE position statement recommends that CDC publish confirmed and probable cases. The CDC’s Mpox Response team is publishing national summaries and aggregate case data during the outbreak. When CDC begins publishing mypox cases in the NNDSS tables, they will include confirmed and probable cases.
If you have questions about sending mpox data to CDC via NNDSS, please email edx@cdc.gov with the subject line “Mpox reporting questions.”
An eSHARE webinar, “Kick-off for annual reconciliation of 2021 NNDSS data and NNDSS collection of final, aggregate 2021 COVID-19 case counts,” was held on August 16, 2022.
Visit the eSHARE archives to access the slides and recordings for past eSHARE webinars. CDC typically posts eSHARE slides and recordings a few days after each webinar.
To join an eSHARE webinar, please see your calendar invitation or contact the CDC Electronic Data Exchange mailbox at edx@cdc.gov for login information with the subject line “eSHARE invitation.”
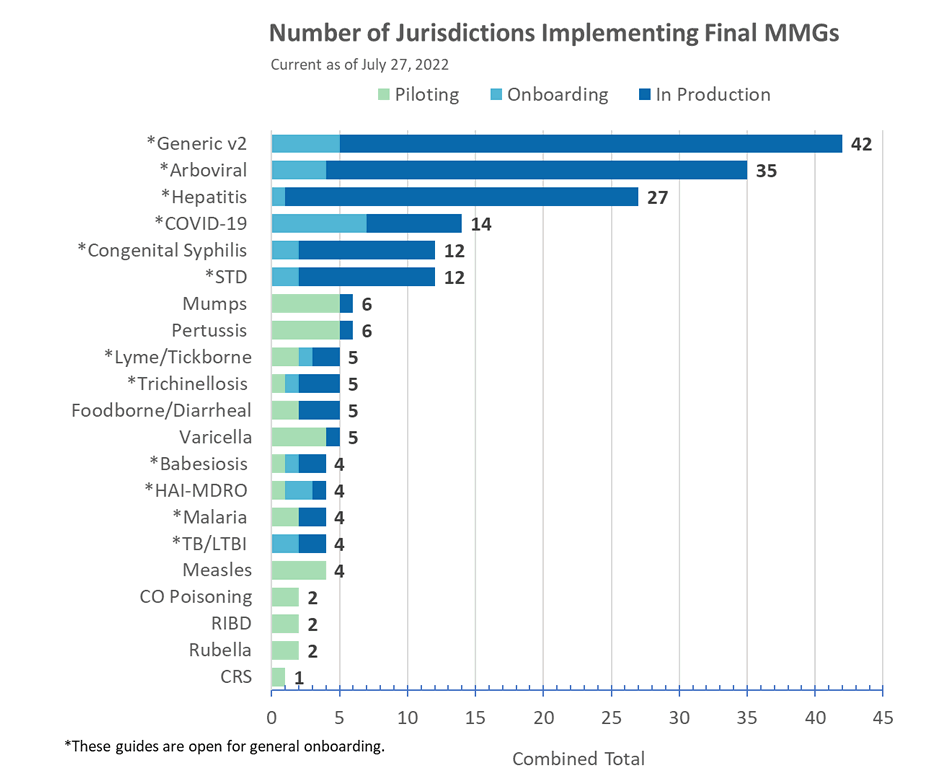
NEW! Babesiosis MMG
Version 1.1 of the Babesiosis MMG has now posted. All value sets associated with this guide are in the PHIN Vocabulary Access and Distribution System (VADS) Babesiosis Case Notification View version 5. The changes include:
- Format description of a value for Physician Phone (68340-9) and
- Addition of one data element, Mother’s Local Record ID (INV2084).
Tuberculosis and Latent TB Infection (LTBI) MMG
Version 3.0.3 of the TB and LTBI MMG has now posted. The changes include various formatting updates and updates to the test case scenario worksheet including two new LTBI scenarios. Additionally, version 7 of the Tuberculosis and LTBI Case Notification View is now available. This view updates the value sets Medication (TB) and Susceptibility Test Type (TB) by correcting a spelling error in preferred concept name “Para-Aminosalicylic acid.”
- Value set Medication (TB) is used for data elements Drug Used to Treat MDR TB (INV1158) and Initial Drug Regimen (INV1143).
- Value set Susceptibility Test Type (TB) is used for data element Antimicrobial Susceptibility Test Type (LABAST6).
If you are interested in joining a cohort or onboarding an MMG, please email the CDC Electronic Data Exchange mailbox at edx@cdc.gov.
Project Update: USDS Case Surveillance Discovery Sprint
In mid-July 2022, a team led by the United States Digital Service (USDS) and in collaboration with CDC completed its discovery sprint to help identify opportunities to modernize the flow of case surveillance data. The team provided a report with its findings and recommendations to CDC in late July 2022, and CDC leadership is currently reviewing the report. CDC expects to provide more details on the report’s results and plans for implementation in coming weeks.
Focus of the sprint
The sprint examined the flow of case reporting and notification. During the sprint, the team reviewed recommendations from prior, independent evaluations and available data and observed processes. It interviewed CDC staff across a range of programs; staff from nine state, territorial, local, and tribal jurisdictions (STLTs); and public health partners such as the National Association of County and City Health Officials and Council of State and Territorial Epidemiologists to gain insights and inform action steps.
The sprint specifically looked at the lifecycle of the data, starting at the point of care and ending in public health action. Its goal was to deepen understanding of the current technologies, processes, and policies around disease surveillance data that will contribute to informing what a modernized, sustainable system could look like.
How the sprint findings will be used
The findings from the sprint will be used to determine how to better support STLTs and CDC programs in data collection and reporting, with an emphasis on minimizing the reporting burden on states. In addition, the sprint findings will help move toward greater flexibility in how CDC receives data from jurisdictions. Sprint findings also will help to ensure greater bidirectionality of data so that states can both see the data they submitted and access analytic and visualization tools that will help them better understand how their data fit into the national picture. Finally, the sprint findings will help develop ways to build up the public health informatics and data science workforce capacity at the local and state levels, including through technical support, training, and innovative approaches to surveillance and staffing.
Building on successes
This sprint has built upon the successes of other previous and ongoing case surveillance improvement efforts, including the extensive work already completed on message mapping guides and data standards. It also is aligned with CDC’s Data Modernization Initiative (DMI) and addresses pre-existing challenges and current opportunities for modernizing the way we work together, and the systems, policies, and processes through which we collect and share public health data.
About USDS
USDS works on technology improvement projects impacting people served by the U.S. government. Since 2014, USDS has partnered with dozens of federal and state agencies to build more than 160 successful digital products. Learn more about how USDS conducts discovery sprints.
If you have any questions about the sprint, please contact Dr. Jennifer Layden, CDC Associate Deputy Director for Public Health Science and Surveillance, at qbg5@cdc.gov and DMI@cdc.gov. Please copy Zuzana Love, USDS Sprint Team, at spy3@cdc.gov, on your e-mail.
Spotlight: Updates to the NNDSS Technical Resource Center Reflect Improved MMG Onboarding Processes
On Thursday, July 21, 2022, the NNDSS team updated the NNDSS Technical Resource Center to share critical information about improved processes for onboarding an HL7 MMG.
Using innovations and lessons learned from the COVID-19 pandemic, CDC’s Data Standardization and Assistance Team streamlined the process for onboarding an MMG. CDC programs, partners such as the Council of State and Territorial Epidemiologists and Association of Public Health Laboratories, and public health jurisdictions all contributed to this important work. These improvements have already made the onboarding process for all MMGs faster and more efficient. They also contribute to the CDC Data Modernization Initiative.
With this update, the NNDSS website now provides the latest information on the streamlined onboarding process. This resource will help jurisdictions better prepare to onboard an MMG and know what to expect during onboarding. In addition, these updates:
- clarify roles and responsibilities during the onboarding process,
- provide guidance to public health jurisdictions interested in transitioning from the generic version 2 (GenV2) MMG to an MMG for a specific condition,
- enhance user experience and site navigation, and
- improve search engine optimization.