July 2021
July 20, 2021: eSHARE call, 3:00–4:00 PM ET
August 2021: Start of reconciliation for 2020 data
October 29, 2021: Deadline to
- sign off on final 2020 reconciliation data
- send final aggregate 2020 coronavirus disease 2019 (COVID-19) case counts to CDC
June 19–23, 2022: Council of State and Territorial Epidemiologists (CSTE) Annual Conference, Louisville, Kentucky
Updated Plan for Reconciliation of 2020 Annual National Notifiable Diseases Surveillance System (NNDSS) Data
Starting in late August 2021, a CDC Data Management and Reporting (DMR) specialist will email each jurisdiction a reconciliation packet. Once a jurisdiction receives their reconciliation packet, a DMR specialist will guide the jurisdiction through the rest of the reconciliation process. The reconciliation packet will include:
- a letter with an overview of the changes to the reconciliation process for 2020 NNDSS data,
- a production calendar (timeline),
- a week-ending calendar for 2020 and 2021,
- an abbreviated event code list for 2020, and
- a copy of the jurisdiction’s reconciliation tables and instructions for reviewing the reconciliation tables.
The process to reconcile 2020 NNDSS data will be similar to previous years, with some exceptions. Jurisdictions will be able to view the processing of all case notification formats in the Message Validation, Processing, and Provisioning System (MVPS) and download a line list of cases for comparison as part of their reconciliation process. The State and Territorial Epidemiologist sign-off process will not be conducted in MVPS this year; sign-off will be done through email, as in previous years.
CDC will offer training about MVPS and the 2020 reconciliation procedures in late August. The deadline for State and Territorial Epidemiologist approval of the final 2020 data is Friday, October 29, 2021.
Process for 2020 Final NNDSS COVID-19 Data
The 2020 final NNDSS COVID-19 data will not be reconciled; however, CDC will collect final 2020 NNDSS aggregate COVID-19 case counts:
- CDC will provide jurisdictions a template and instructions.
- Jurisdictions will submit their final aggregate case counts in the template.
- State and Territorial Epidemiologists will sign off on the aggregate 2020 COVID-19 case counts.
- CDC will use aggregate case counts in the 2020 NNDSS annual tables.
The tables will include confirmed, probable, and total cases of COVID-19 among U.S. residents, with stratifications by jurisdiction of residence, sex, age group, race, ethnicity, and month (based on MMWR week).
Jurisdictions should plan to send their final aggregate 2020 COVID-19 case counts to CDC by October 29, 2021, which is the same deadline for submitting final 2020 data for other NNDSS conditions.
July’s Topic: CSTE 2021 Virtual Conference Highlights
Mark your calendar for the next NNDSS eSHARE session, scheduled for Tuesday, July 20, 2021, from 3:00-4:00 PM ET, which will feature highlights from the 2021 CSTE Virtual Conference.
To join a webinar, please see your Outlook invitation or contact the CDC Electronic Data Exchange mailbox at edx@cdc.gov for login information.
Message Mapping Guides (MMGs) Updated to Reflect new NNDSS Website
The NNDSS team is updating all MMGs with new web addresses to reflect the new NNDSS website. Although these updates ensure that users can easily link to needed information, they do not impact the message format or content. The updated MMGs will be identified by an increment in the third component of the version number. The Generic v.2.0.1 MMG will also incorporate the new data element prioritization format (R,1,2,3).
COVID-19 Message Mapping Guide Updated
CDC posted an updated COVID-19 MMG (v1.0.1), the “COVID Lite” test case scenarios worksheet (TCSW), and associated test scenarios to the NNDSS website. The COVID-19 MMG v1.0.1 TCSW tab now contains vaccine information that aligns with the values present in the “COVID Lite” TCSW. Both documents in the TCSW tab now align with the same test values. Additionally, COVID-19 v.1.0.1 includes formatting updates being applied across all guides, including:
- updated URLs to link to the new NNDSS and new MMG websites,
- updated language to support version 3 series PHIN Messaging Specification guides for case notifications, and
- updated column description for “May Repeat” to provide additional information on “Y/#.”
Tuberculosis (TB) and Latent TB Infection MMG Updated
The PHIN Vocabulary Access and Distribution System TB and Latent TB Infection Case Notification View version 6 is now available. It includes additional values for Gene Name (TB).
If you are interested in joining a cohort or onboarding an MMG, please email the CDC Electronic Data Exchange mailbox at edx@cdc.gov.
NNDSS Updates and Insights from the 2021 CSTE Virtual Conference
At the recent CSTE virtual conference held June 13–17, 2021, CDC staff provided important updates and insights about NNDSS. They also gathered critical feedback from public health jurisdictions and key partners to help inform future work on improving NNDSS. In this article, you can find brief summaries and key insights for each NNDSS-related presentation along with links to presentation materials.
If you have additional feedback or questions for the NNDSS team, email us at edx@cdc.gov. We encourage everyone to mark their calendars for the next CSTE Annual Conference, which will be held June 19–23, 2022, in Louisville, Kentucky!
“Developing Data Quality Reports or Tools at CDC for Jurisdictions to Use to Monitor Data Submitted to NNDSS,” a roundtable moderated by Delicia Carey and Ruth Jajosky, CDC Case-based Surveillance Operations Team, focused on gathering feedback from jurisdictions on how they currently monitor NNDSS data quality and on the types of data quality tools CDC could develop to help jurisdictions fill gaps in their current processes.
Key insights:
- Jurisdictions indicated it would be useful for CDC to issue data outage alerts when CDC does not receive data from a jurisdiction for a defined period. Some jurisdictions indicated CDC should allow for configurable data outage thresholds for jurisdictions to set themselves. Other jurisdictions expressed interest in having thresholds that were harmonized across jurisdictions.
- Jurisdictions felt data quality reports generated by CDC would help jurisdictions review their data and could help facilitate data reconciliation.
- Jurisdictions may want to filter their data quality reports on not only individual conditions (event codes) but also a group of conditions.
- Some jurisdictions expressed interest in continuing to be involved in future discussions CDC has about data quality reports. If you are interested, email Delicia Carey (ex0@cdc.gov) and Ruth Jajosky (raj3@cdc.gov).
Roundtable Handout [169 KB, 4 Pages, 508]
“Increasing Efficiency of Onboarding for State and Local Public Health Agencies Sending NNDSS HL7 Case Notifications,” a roundtable moderated by Danielle Tack and Michele Hoover, CDC Data Standardization and Assistance Team, asked jurisdictions how CDC can increase the efficiency in onboarding, resources, and technical assistance for sending NNDSS case notifications.
Key insights:
- Harmonization across guides is helpful, but the timing of case report form updates will determine if it will speed up the pre-onboarding process. Gap analysis and mapping should inform future efforts.
- Implementing automation in reviewing messages can help improve the time needed to identify issues and onboard MMGs.
- A summary of common onboarding errors or warnings would help with message review prior to sending.

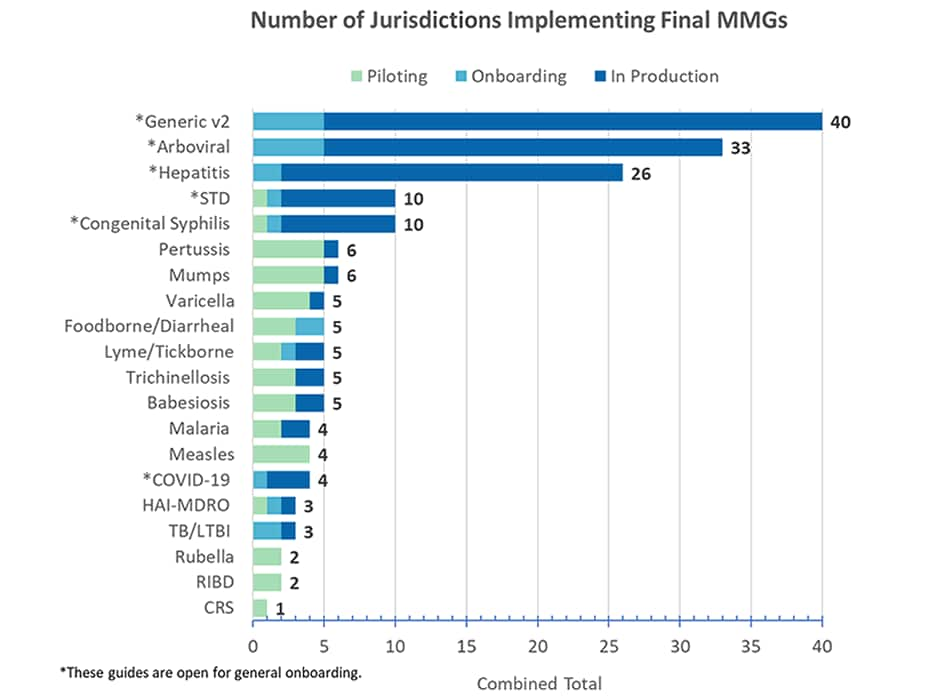
“NNDSS Progress and Future Directions,” presented by Sara Johnston, NNDSS Program Lead, provided a recap of NNDSS’ COVID-19 response activities in 2020 and 2021, including how NNDSS rapidly developed a new COVID-19 MMG in 2 months and quickly ramped-up processing to handle the unprecedented volume of cases submitted to NNDSS. The presentation also provided an update on upcoming enhancements to MVPS. Once completed, these enhancements will consolidate processing for various NNDSS data sources into MVPS and provide new functionality to help jurisdictions review their data, delete cases or groups of cases, verify low-incidence conditions, and perform reconciliation activities. Finally, the presentation highlighted the new NNDSS website and Case Surveillance News newsletter (formerly NMI Notes), gave an update on the status of MMG production, and emphasized the importance of NNDSS work to meet the goals of the CDC Data Modernization Initiative.
Presentation Slides [13 MB, 20 Slides, 508]
“Working Together to Promote Jurisdiction Efficiencies to Send COVID-19 NNDSS Case Notifications,” a presentation by Michele Hoover, CDC Data Standardization and Assistance Team, described the process used to increase jurisdictions’ ability to send timely COVID-19 data to CDC using the Gen v2 and COVID-19 MMGs. CDC collaborated with the Association of Public Health Laboratories and the National Electronic Disease Surveillance System Base System team to improve current technical assistance and onboarding processes. By identifying potential barriers, challenges, and lessons learned, the project decreased onboarding time. This significant achievement was possible through collaboration among CDC, jurisdictions, and key partners.
Presentation Slides [1 MB, 20 Slides, 508]
“Developing Visualization Tools for CDC Disease Programs and Jurisdictions to Monitor Diseases Using the National Notifiable Diseases Surveillance System,” a roundtable moderated by Michelle Lin and Yusheng Zhai, CDC Case-based Surveillance Operations Team, focused on understanding how jurisdictions currently visualize NNDSS data and obtaining input to develop and refine interactive visualization tools that will help jurisdictions better visualize their data.
Key Insights:
- Data cleaning is an important step in preparing data for analytics and visualization.
- Jurisdictions use a wide variety of visualization tools, including Power BI, ESSENCE tools, Excel, SAS, and Tableau.
- Jurisdictions expressed interest in having CDC share code for visualization and automation of visualizations, as has been done with syndromic surveillance. An example from syndromic surveillance is using R shiny and R code to automate visualizations.
- Jurisdictions would find it helpful to be able to compare their data to regional or national trends.
- In a discussion about data sharing agreements across jurisdictions, several challenges were mentioned. Some conditions are so unique that sharing data is difficult without violating data sharing agreements.