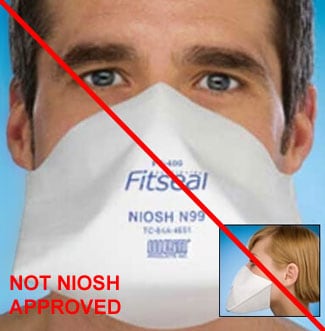
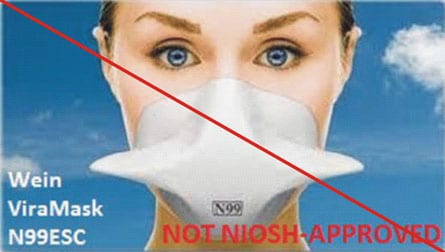
Counterfeit Respirators / Misrepresentation of NIOSH Approval
Notice on HHS Ownership of Respirator Certification Marks
NIOSH, on behalf of the Department of Health and Human Services (HHS), registered several certification marks with the U.S. Patent and Trademark Office (USPTO) that are specific to NIOSH Approved® respirators. These registered certification marks currently include the NIOSH stylized logos with and without full text, NIOSH Approved®, and certification marks, such as N95® and P100®, that correspond to the approved respiratory protection levels for powered and non-powered air-purifying respirators (APRs). The registered certification marks can be found on the NIOSH Post-market Evaluations Conducted by NIOSH webpage under the section Protecting End Users from Wearing Respirators Being Misrepresented as NIOSH Approved® Respirators. Only manufacturers who are NIOSH approval holders may use these registered certification marks. Any misuse of the aforementioned marks is a direct violation of applicable trademark law and may be subject to enforcement action. For more information, view the NIOSH Conformity Assessment Letter to Manufacturers, NIOSH CA 2023-1056.
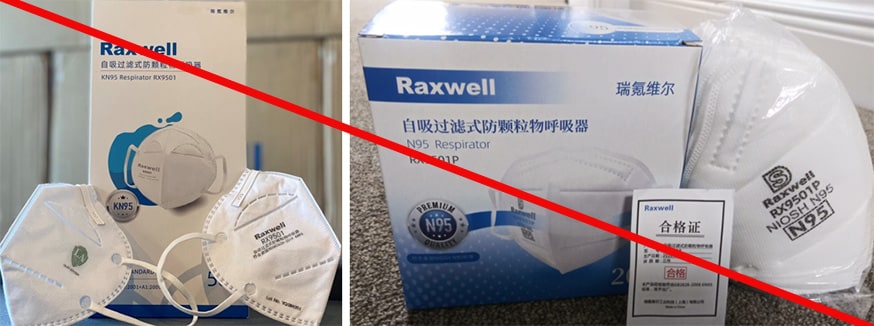
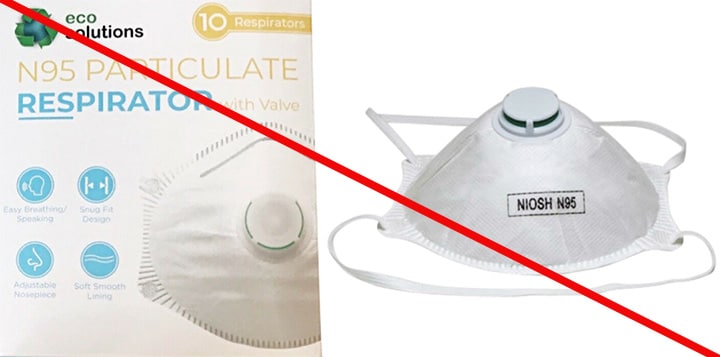
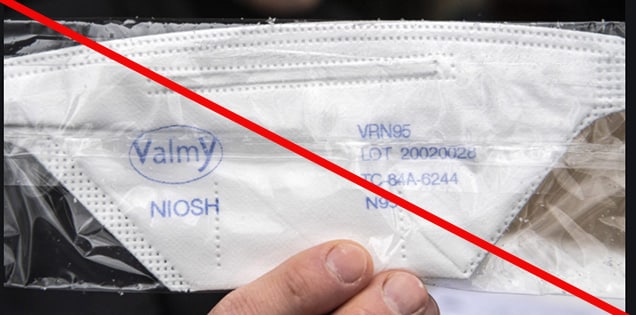
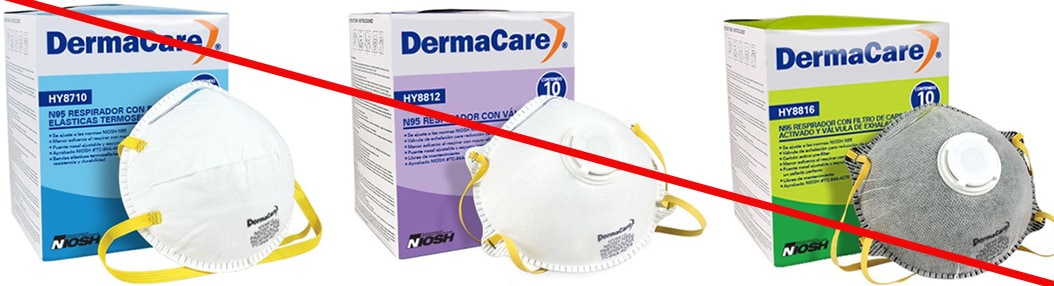
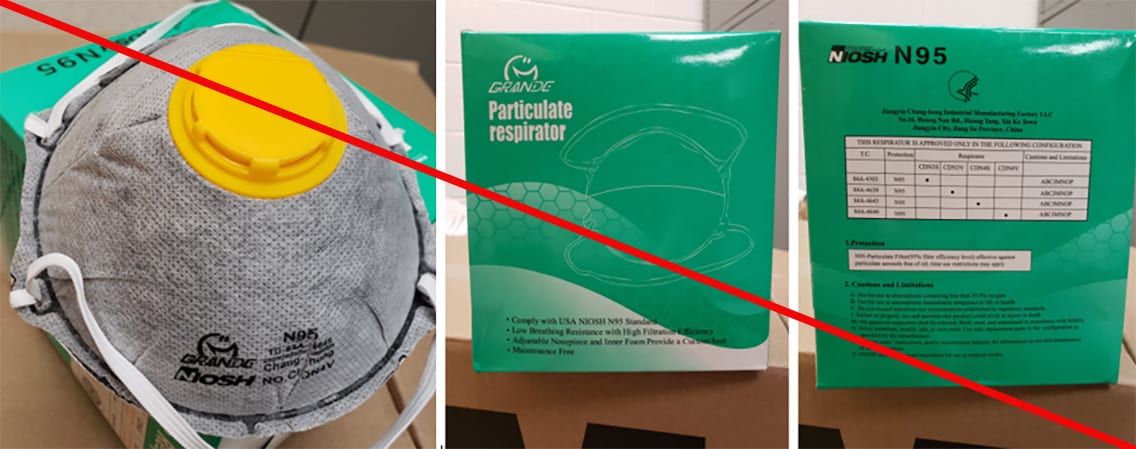
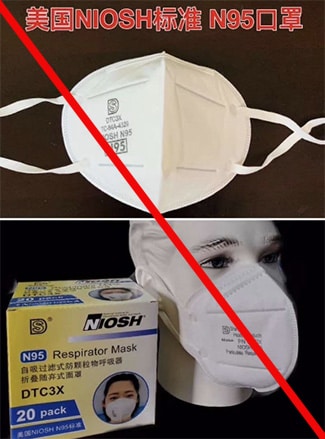
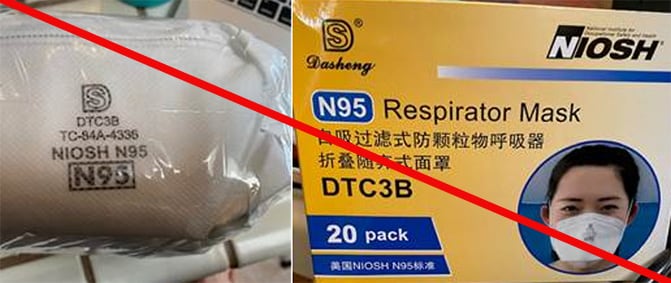
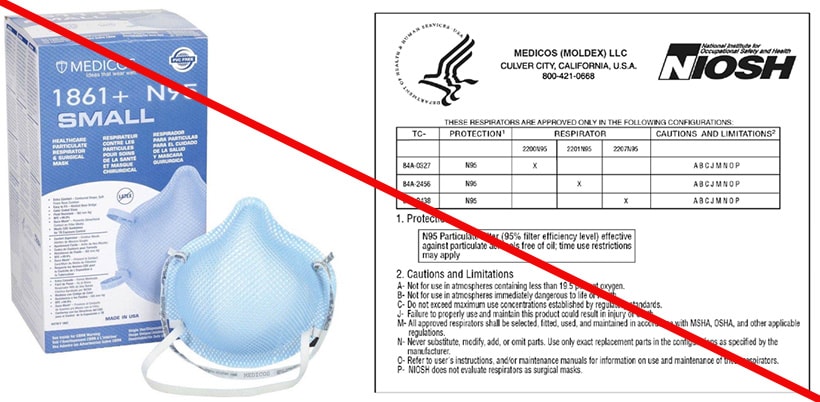
Misrepresented respirators include all respirators that are falsely marketed and sold as NIOSH Approved respirators when they are not. Counterfeit respirators specifically refer to products trying to copy an actual NIOSH Approved model. Both counterfeit and misrepresented respirators may not be capable of providing the appropriate or necessary respiratory protection to workers and users. NIOSH posts information about misrepresented and counterfeit respirators here to alert users, purchasers, and manufacturers.
Learn more about how to identify NIOSH Approved respirators and counterfeits. View examples of counterfeit or misrepresented respirators below.
How to identify a NIOSH Approved respirator:
All NIOSH Approved respirators have a testing and certification (TC) approval number (e.g., TC 84A-XXXX). The NIOSH approval label, which you can find on or within the respirator packaging, includes the TC approval number. Additionally, an abbreviated approval label is on the filtering facepiece respirator (FFR) itself or straps. NIOSH Approved FFRs will always have one of the following designations:
- N95
- N99
- N100
- R95
- R99
- R100
- P95
- P99
- P100
You can verify a TC approval number is valid by checking the NIOSH Certified Equipment List. More information is available on the Respiratory Protection Information Trusted Source.
To learn more about how to identify a NIOSH Approved FFR, check out the fact sheet and graphic below.
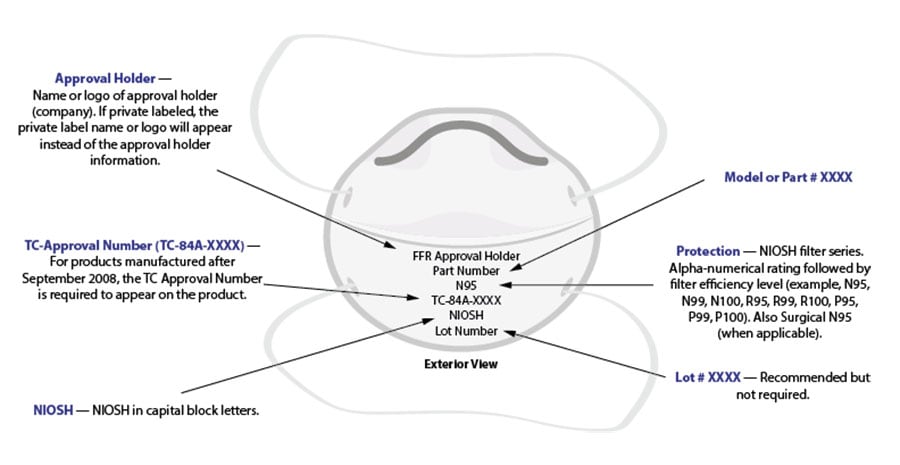
Example of correct exterior markings on a NIOSH Approved filtering facepiece respirator. View larger version.
How to Tell if Your N95® Respirator is NIOSH Approved
NIOSH Approved N95 respiratory protective devices are the most common type of filtering facepiece respirator (FFR) used in U.S. workplaces. Our video explains how to determine if an N95 FFR is NIOSH approved, including required labeling and where to look to confirm an approval number. It also provides tips for recognizing counterfeit and misrepresented respirators.
Signs that a respirator may be counterfeit:
- No markings at all on the filtering facepiece respirator
- No approval (TC) number on filtering facepiece respirator or headband
- No NIOSH markings
- NIOSH spelled incorrectly
- Presence of decorative fabric or other decorative add-ons (e.g., sequins)
- Claims approval for children (NIOSH does not approve any type of respiratory protective device for children at this time)
- Filtering facepiece respirator has ear loops instead of headbands. At this time, NIOSH has not approved respirators that use ear loops without the use of an approved fastener. The fastener connects the loops behind the head.
Check out more tips to spot counterfeit respirators.

Buyer Beware! NIOSH has identified many combination chemical/filter cartridges being sold on well-known online marketplaces claiming to have chemical and P100® protection that are not part of a NIOSH Approved® respirator configuration and are misusing the registered P100 certification mark. Such cartridges and respirators have not undergone NIOSH’s rigorous review process and may not provide the claimed protection level and may pose a risk to the user.
The examples above are counterfeit combination cartridges using the same part numbers (e.g., 60921, 60923, 60924 and 60926) associated with authentic 3M combination cartridges that are part of a NIOSH Approved configuration. For example, many listings incorrectly or falsely claim these cartridges can be used with the 3M 6000, 7000 and FF-400 series facepieces.
One way to identify counterfeit chemical cartridge/filters is by the missing information on the abbreviated label. The abbreviated label (i.e., markings) on cartridge/filters that are part of a NIOSH Approved configuration must indicate:
- the approval holder’s name,
- product part/model number,
- protections/filter series,
- the acronym “NIOSH”, and
- lot number
Please Note: Mandatory information is missing from the counterfeit cartridges shown in the photo examples above. However, all of the information appears on the genuine cartridges shown below.

Some manufacturers/sellers that are not NIOSH approval holders may claim their cartridges/filters are compatible with facepieces manufactured by NIOSH approval holders. Users cannot use these products in place of the cartridge/filter component associated with the NIOSH Approved respirator. If so, it will void the NIOSH approval and may not provide the claimed level of protection to the user.

Buyer Beware! NIOSH has identified many filters sold on well-known online marketplaces claiming to be P100® filters that are not part of a NIOSH Approved® respirator configuration.
The examples above are counterfeit filters using the same part numbers associated with authentic NIOSH Approved 3M P100 filters. For example, many listings incorrectly or falsely claim these filters can be used with the 3M 6000 series facepieces.
One way to identify counterfeit P100 filters is by the missing information on the filters. The abbreviated label (i.e., markings) on NIOSH Approved P100 filters must indicate:
- the approval holder’s name,
- product model or trade name,
- protections/filter series,
- part number,
- the mark “NIOSH”, and
- lot number (location varies; could be on filter, packaging, user instructions)
Note that some or most of this mandatory information is missing from the counterfeit filters shown in the examples above. However, all of this information appears on the genuine NIOSH Approved P100 filters (examples to the right).

Some manufacturers/sellers that are not NIOSH approval holders may claim their filters are compatible with facepieces manufactured by NIOSH approval holders. Users cannot use these filters in place of the filter component associated with the NIOSH Approved respirator. If so, it will void the NIOSH approval and may not provide the claimed level of protection to the user.

Breath Buddy is NOT a NIOSH approval holder. They are falsely indicating product can be used with half and full facepieces made by other NIOSH approval manufacturers. The Breath Buddy Particulate Filter is NOT a component associated with a NIOSH approval. Users cannot use this filter in place of the filter component associated with the NIOSH Approved respiratory protective device. If so, it will void the NIOSH approval. (1/26/2022)

Chengde Technology Co., Ltd. is misusing NIOSH test information regarding Gosbuy KN95 face masks. The company references NPPTL testing claims , using an image of the test setup from the assessment. The NIOSH website states manufacturers, distributors, suppliers, and importers cannot use these results to make claims about their products. They cannot use the results to influence purchasers or make claims that the product meets NIOSH approval requirements. Chengde Technology Co., Ltd. is not a NIOSH approval holder or a private label assignee. (1/18/2022)

Good Mask Co. is misusing NIOSH test information regarding the “Good Folding KN95” mask; marketing as “CDC-approved, NIOSH-certified.” This statement is misleading because CDC, through NIOSH, does not approve KN95 masks. Nor does NIOSH approve any other respiratory protective device solely certified to international standards. Additionally, Good Mask Co. is misusing NIOSH test information. The product package indicates it meets Chinese standard GB 2626-2006 and submitted to NIOSH under an International Respirator Assessment request. Good Mask Co. markets the mask using results from the assessment. The NIOSH website states manufacturers, distributors, suppliers, and importers cannot use to make claims about their products. They cannot use the results to influence purchasers or make claims that the product meets NIOSH approval requirements. Huizhou Green Communication Equipment Manufacturing Co., Ltd. is not a NIOSH approval holder or a private label assignee. (1/13/2022)
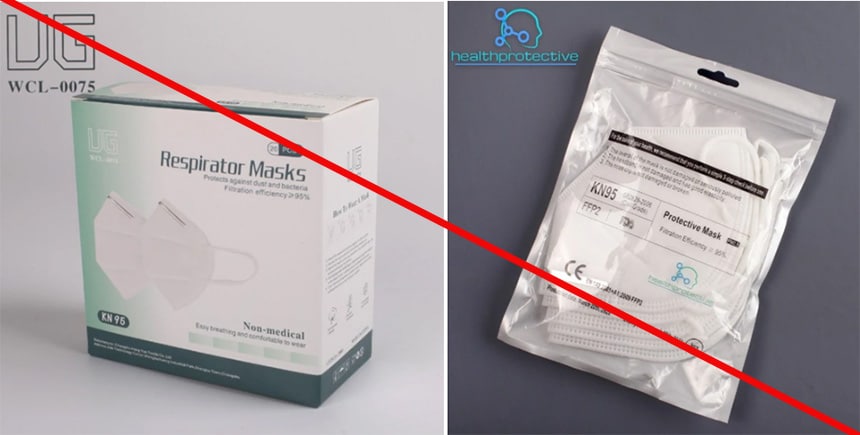
Health Protective is marketing a KN95 mask as “Certified KN95 respirator mask, adopted by the CDC.” This statement is misleading because CDC, through NIOSH, does not approve KN95 masks. Nor does NIOSH approve any other respiratory protective device solely certified to international standards. Additionally, Health Protective is misusing NIOSH test information. The product package indicates it meets Chinese standard GB 2626-2006 and submitted to NIOSH under an International Respirator Assessment request. Health Protective advertises the KN95 mask using results from the assessment. The NIOSH website states manufacturers, distributors, suppliers, and importers cannot use these results to make claims about their products. hey cannot use the results to influence purchasers or make claims that the product meets NIOSH approval requirements. Changshu City Hengyun Nonwoven Products Co., Ltd. is not a NIOSH approval holder or a private label assignee. (1/11/2022)
View Additional Counterfeit or Misrepresented Respirators Listed from Previous Years
If you suspect you have a counterfeit or misrepresented respirator, contact NIOSH at ppeconcerns@cdc.gov.
Please provide the following details with your email:
- Any manufacturer names present on the respirator
- Respirator model or part number
- Photos of the respirator and packaging
- NIOSH approval number (e.g., TC 84A-XXXX), if present
- Web url where respirator was purchased or found
The NIOSH stylized logos (shown below), N99, N100, R95, P95, P100, PAPR100-N, PAPR100-P, and HE are certification marks of the U.S. Department of Health and Human Services (HHS) registered in the United States.

N95 and NIOSH Approved are certification marks of the U.S. Department of Health and Human Services (HHS) registered in the United States and several international jurisdictions.