NIOSH Conformity Assessment Letter to Manufacturers
This notice has been superseded. For the latest NIOSH guidance on this topic, please see the updated notice: https://www.cdc.gov/niosh/npptl/resources/pressrel/letters/conformitymanuf/CA-2018-1010-R1.html
NIOSH CA 2018-1010
November 2018
Summary
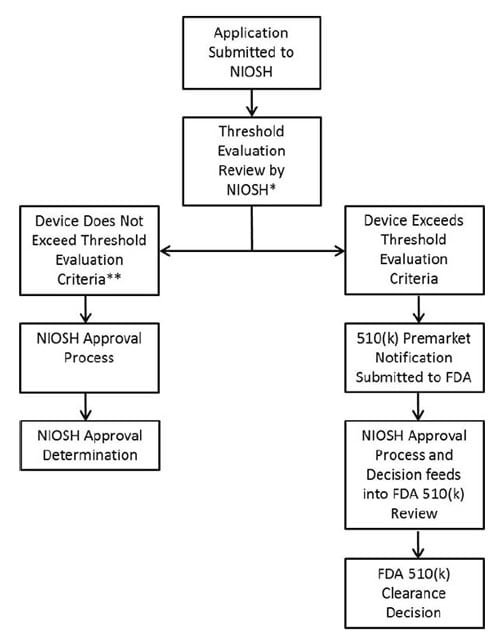
Beginning December 1, 2018, the National Institute for Occupational Safety and Health (NIOSH) will accept applications to implement the coordinated regulatory process to exempt a subset of filtering facepiece respirators (FFRs) from Food & Drug Administration (FDA) premarket notification requirements. The N95 FFRs and data provided by the applicant must demonstrate:
- all applicable NIOSH N95 FFR requirements found in 42 CFR 84;
- flammability, fluid resistance, and biocompatibility, previously reviewed by the FDA, and evaluated by NIOSH in accordance with the Memorandum of Understanding (MOU) between the FDA/Center for Devices & Radiological Health and the Centers For Disease Control & Prevention/NIOSH/National Personal Protective Technology Laboratory when FDA Threshold Criteria is not exceeded;
- no significant deviation from respirator designs previously cleared by the FDA, such as the addition of exhalation valves.
Any device approved under this Guidance will be labeled with a protection of “N95-F.” This protection indicates that the NIOSH-approved device meets the intended flammability, fluid resistance, and biocompatibility requirements and can be used in healthcare settings. Monthly NIOSH Certified Equipment List updates will include searchable information about approved N95-F respirators.
Additionally, N95-F respirator approval labels will have a new caution and limitation “WW” defined as: This respirator conforms to recognized standards for biocompatibility, flammability, and fluid resistance. The approval label is no longer required to have the caution and limitation “P” – NIOSH does not evaluate respirators for use as surgical masks.
Four specific situations are noted:
- No further action under this Guidance is required for an existing device that has previously obtained NIOSH approval and FDA 510(k) clearance (i.e., surgical N95 FFRs). The existing approval will remain effective as long as the approval holder continues to 1) pay the annual NIOSH maintenance fee, 2) list the device with the FDA, and 3) meet NIOSH and FDA post-market requirements.
- If a manufacturer of an existing NIOSH-approved N95 FFR respirator has not previously sought FDA 510(k) clearance and now seeks to label this device with the additional (flammability, fluid resistance, and biocompatibility) protections the manufacturer must follow this Guidance to achieve a new NIOSH approval for the N95-F respirator.
- If a manufacturer of an existing NIOSH-approved N95 FFR respirator has not previously sought FDA 510(k) clearance and does not seek to label this device with the additional (flammability, fluid resistance, and biocompatibility) protections, the existing NIOSH approval will remain effective.
- If a manufacturer of a new N95 FFR seeks NIOSH approval with these additional protections, the manufacturer must follow this Guidance.
NEW NIOSH APPLICANTS:
If an applicant has never previously submitted any type of respiratory protective device for NIOSH approval, the applicant must first apply to NIOSH for a three-character Manufacturer’s Code by completing the Prospective Approval Holder Form and returning it to the NIOSH NPPTL Records Room. Obtain this form by contacting the NIOSH NPPTL Records Room at recordsroom@cdc.gov. After obtaining the Manufacturer’s Code, the manufacturer seeking approval for an N95-F must follow this Guidance.
NEW AND EXISTING NIOSH APPLICANTS:
The applicant will use the NIOSH Standard Application Procedure (SAP) to complete the Standard Application Form. When completing the reason for application section (C.9), the applicant will indicate they are using the consolidated process and seeking approval within the terms of the FDA Final Order and the MOU.
The approval label provided as part of the NIOSH application must include the new caution and limitation “WW” defined as: This respirator conforms to recognized standards for biocompatibility, flammability, and fluid resistance.
Applicants are required to provide documentation to NIOSH to indicate conformance to the FDA thresholds for flammability, fluid resistance, and biocompatibility. All data must be received in electronic formats as indicated in the NIOSH SAP. While NIOSH completes testing of hardware in accordance with 42 CFR 84 Subpart K, NIOSH is not conducting testing to verify the flammability, fluid resistance, or biocompatibility performance of the respirator as part of the N95-F approval process.
During the NIOSH document review process, NIOSH will review flammability, fluid resistance, and biocompatibility test data and results provided by the applicant and in accordance with the FDA Threshold Criteria.
In accordance with the MOU, N95-F Approval Holders are required to fulfill the general control provisions, under section 510 of the FD&C Act, including annual registration and listing obligations.
Claims exceeding the threshold criteria defined in the MOU will result in a denied application for N95-F approval (MOU Appendix, Section 2). A manufacturer making such claims and seeking approval must apply using the current, pre-MOU, process for approval by NIOSH, as an N95 FFR, and clearance by the FDA, with finalization of the labeling by NIOSH. The FDA will continue to be responsible for the review of 510(k) submissions for N95 FFRs regulated under 21 CFR 878.4040 that exceed the conditions and limitations of exemption outlined within the Final Order and ensuring manufacturers comply with applicable regulations.
Note: Tuberculosis protection claims in accordance with CDC Guidance are allowed. Applicants are reminded to consider FDA recommendations for labeling medical products to inform users that the product is not made with natural rubber latex.
NIOSH is developing a methodology for conducting product audit evaluations of NIOSH-approved N95-F FFRs. These product audit evaluations will include NIOSH assessment of the flammability and fluid resistance performance.
REFERENCES
FDA Final Order
FDA Postmarket Requirements (Devices)
Approval of Respiratory Protective Devices, 42 C.F.R, Part 84
MOU 225-18-006 (also included below)
Reference: MEMORANDUM OF UNDERSTANDING (MOU) 225-18-006), November 2017