Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail.
HIV Testing and Treatment Among Tuberculosis Patients --- Kenya, 2006--2009
In resource-limited settings, high case-fatality rates are seen among tuberculosis (TB) patients with human immunodeficiency virus (HIV) infection, especially during the early months of TB treatment (1). HIV prevalence among TB patients has been estimated to be as high as 80%--90% in some areas of sub-Saharan Africa (2). In 2004, the World Health Organization (WHO) recommended increasing collaboration between HIV and TB programs (3). Since then, many countries, including Kenya, have worked to increase TB/HIV collaborative activities. In 2005, the Kenya Division of Leprosy, Tuberculosis, and Lung Disease (DLTLD) added questions regarding HIV testing and treatment to the existing TB surveillance system.* This report summarizes HIV data collected from Kenya's extended TB surveillance system during 2006--2009. During this period, HIV testing among TB patients increased from 60% in 2006 to 88% in 2009, and the prevalence of HIV infection among TB patients tested decreased from 52% to 44%. In 2009, 92% of HIV-infected TB patients received cotrimoxazole prophylaxis for the prevention of opportunistic infections (4). Although these data highlight the increase in HIV services provided to TB patients, only 34% of HIV-infected TB patients started antiretroviral therapy (ART) while being treated for TB. Innovative interventions are needed to increase HIV treatment among TB patients in Kenya, especially considering the 2009 WHO guidelines recommending that all HIV-infected TB patients be started on ART as soon as possible, regardless of CD4 count (5). Although these guidelines have not yet been implemented in Kenya, officials are working to identify methods of increasing access to ART for TB patients.
In 2004, the Kenya Ministry of Health (which in 2008 became the Ministry of Public Health and Sanitation [MOPHS]) established the TB/HIV Coordinating Committee to help develop policy and guidance for implementation of TB/HIV collaborative activities. The committee recommended using the existing national TB program infrastructure to expand HIV counseling and testing services† to TB patients. In addition, the committee recommended using provider-initiated testing and counseling, an "opt-out" model in which HIV testing is performed routinely unless the patient declines. Because cotrimoxazole prophylaxis has been shown to reduce opportunistic infections and to decrease morbidity and mortality for HIV-infected TB patients, the committee recommended that TB clinics offer cotrimoxazole prophylaxis to all HIV-infected TB patients (i.e., those with documentation of a positive HIV test result in the facility TB register) (6). Finally, the committee recommended that HIV-infected patients be referred to separate HIV care and treatment clinics for additional HIV care and evaluation for eligibility for ART.§
DLTLD is responsible for overseeing clinical activities at approximately 2,200 TB diagnostic and treatment facilities and for collecting routine surveillance data. Provincial and district TB/leprosy coordinators manage the network of TB facilities. District coordinators receive quarterly reports regarding all patients with active TB disease who are newly registered (i.e., currently diagnosed with active TB disease and receiving TB treatment) at each TB clinic, compile this information into quarterly aggregate district reports, and then forward the reports to the provincial coordinators, who submit the information to DLTLD.
In 2005, DLTLD added key HIV-related information to the local TB facility register and the district-level reporting forms: HIV testing status for TB patients, HIV test results, and receipt of cotrimoxazole prophylaxis, which are available directly from TB clinic records, and information about ART during TB treatment, which generally is based on patient reports of care received at separate HIV clinics (8). By January 1, 2006, all TB districts in Kenya had added these HIV variables to the routine TB surveillance reporting forms. For this report, data collected through the extended TB surveillance system during 2006--2009 were analyzed.
From 2006 to 2009, the total number of newly registered TB patients reported each year decreased 5%, from 115,234 to 110,015 (Table). The prevalence of HIV testing among TB patients increased from 60% (of 115,234 patients) to 88% (of 110,015 patients), and the prevalence of HIV infection among TB patients tested decreased from 52% (of 69,337 tested) in 2006 to 44% (of 96,280 tested) in 2009. In 2009, HIV prevalence among TB patients varied widely by province, ranging from 5% in North Eastern Province to 70% in Nyanza Province (Figure).
Provision of cotrimoxazole prophylaxis to HIV-infected TB patients remained high throughout this period; 87% received cotrimoxazole in 2006, and 92% in 2009. During the same period, the percentage of HIV-infected TB patients receiving ART increased from 26% to 34% (Table).
Reported by
J Sitienei, MD, H Kipruto, Kenya Div of Leprosy, Tuberculosis, and Lung Disease, Ministry of Public Health and Sanitation. L Nganga, MD, M Ackers, MD, J Odhiambo, MD, Global AIDS Program (Kenya). K Laserson, ScD, Center for Global Health, Kenya. AK Nakashima, MD, Global AIDS Program (Atlanta); S Modi, MD, EIS Officer, CDC.
Editorial Note
Within 5 years of the addition of HIV activities to the country's TB program, 88% of TB patients in Kenya were tested for HIV, and 92% of HIV-infected TB patients received cotrimoxazole prophylaxis in TB clinical settings. Elsewhere in sub-Saharan Africa, success with HIV testing of TB patients varies widely; Malawi tests approximately 80% of TB patients, but estimates of testing are lower in Uganda (60%), Zambia (60%), and South Africa (40%) (CDC, unpublished data, 2010).
HIV testing and clinical services in Kenya historically have been provided through the National AIDS and STI Control Programme. However, the findings in this report show that DLTLD has been successful in providing key HIV services within the existing TB program infrastructure. Multiple actions were critical to achieving this success, including establishment of the TB/HIV Coordinating Committee, which assisted with development of national guidelines for HIV testing in 2004 and promoted provider-initiated testing and counseling in multiple health-care settings (7). Provider-initiated testing and counseling has been shown to increase the proportion of patients tested when compared with traditional "opt-in" models in which patients must request HIV testing (2). As HIV testing among newly registered TB patients increased, the prevalence of HIV among TB patients decreased, indicating that providers might have targeted early testing efforts to patients at greater risk for HIV (8). Overall, HIV prevalence among newly registered TB patients remains high, particularly in Nyanza Province (70%).
In addition to strong commitment to TB/HIV collaborative activities at the national level in Kenya, local leaders have been recruited to form regional TB/HIV coordinating bodies to translate national policy into action. These regional bodies implemented continuing medical education modules to promote provider-initiated testing and counseling and cotrimoxazole prophylaxis for HIV-infected patients as standard interventions in all TB clinical settings. Financial support from international donors including the U.S. President's Emergency Plan for AIDS Relief (PEPFAR), WHO, and the Global Fund to Fight AIDS, Tuberculosis, and Malaria also has been critical to the success of TB/HIV collaborative efforts. This funding has allowed MOPHS to hire additional staff members to support TB/HIV collaborative activities, and to ensure an uninterrupted supply of HIV rapid test kits, cotrimoxazole prophylaxis, ART, and monitoring and evaluation tools.
Despite these efforts, provision of ART to persons with HIV during TB treatment remains at only 34%. Data from the region indicate that more than 90% of HIV-infected TB patients in Kenya likely meet the country's CD4 count criteria for initiating ART (8), underscoring a large unmet need for treatment in this population.
The findings in this report are subject to at least two limitations. First, the number of HIV-infected TB patients receiving ART might have been underestimated. Some HIV-infected TB patients might have received ART late in TB treatment or after the end of TB treatment, and this information might not be captured by the extended TB surveillance system. No formal mechanism exists for transmitting information from the HIV clinic that provides ART to the TB clinic that reports these data. Second, this report relies on surveillance data, which often are subject to reporting delays and might not reflect the most recent program performance.
Initiation of ART for persons with HIV during TB treatment has been shown to reduce mortality by approximately 50% (9). In 2009, WHO recommended that all HIV-infected TB patients be started on ART regardless of CD4 count (5). Although Kenya's ART-eligibility criteria have not yet been changed, MOPHS has been working to identify methods of increasing access to ART for TB patients. Integration of HIV testing and cotrimoxazole provision into TB clinics in Kenya has resulted in increases in testing and cotrimoxazole prophylaxis. Similar increases might result with ART if offered within the TB clinic and not at another clinical site. One high-volume TB clinic in rural Kenya has integrated provision of ART into the clinic, resulting in a fourfold increase in ART initiation among HIV-infected TB patients (10). Additional strategies are needed to improve access to ART and strengthen linkages between TB clinics and HIV clinics to improve outcomes for HIV-infected TB patients.
References
- CDC. Mortality among patients with tuberculosis and associations with HIV status---United States, 1993--2008. MMWR 2010;59:1509--13.
- CDC. Provider-initiated HIV testing and counseling of TB patients---Livingstone District, Zambia, September 2004--December 2006. MMWR 2008;57:285--9.
- World Health Organization. Interim policy on collaborative TB/HIV activities. Geneva, Switzerland: World Health Organization; 2004. Available at http://whqlibdoc.who.int/hq/2004/who_htm_tb_2004.330_eng.pdf. Accessed November 22, 2010.
- World Health Organization. Guidelines on co-trimoxazole prophylaxis for HIV-related infections among children, adolescents, and adults: recommendations for a public health approach. Geneva, Switzerland: World Health Organization; 2006. Available at http://www.who.int/entity/hiv/pub/guidelines/ctxguidelines.pdf. Accessed November 22, 2010.
- World Health Organization. Rapid advice: antiretroviral therapy for HIV infection in adolescents and adults. Geneva, Switzerland: World Health Organization; 2009. Available at http://www.who.int/entity/hiv/pub/arv/rapid_advice_art.pdf. Accessed November 22, 2010.
- Chakaya JM, Mansoer JR, Scano F, et al. National scale-up of HIV testing and provision of HIV care to tuberculosis patients in Kenya. Int J Tuberc Lung Dis 2008;12:424--9.
- Republic of Kenya Ministry of Health. National AIDS and STI Control Programme: guidelines for HIV testing in clinical settings. Nairobi, Kenya: Republic of Kenya Ministry of Health; 2004.
- Teck R, Ascurra O, Gomani P, et al. WHO clinical staging of HIV infection and disease, tuberculosis, and eligibility for antiretroviral treatment: relationship to CD4 lymphocyte counts. Int J Tuberc Lung Dis 2005;9:258--62.
- Abdool Karim SS, Naidoo K, Grobler A, et al. Timing of initiation of antiretroviral drugs during tuberculosis therapy. N Engl J Med 2010;362:697--706.
- Huerga H, Spillan H, Guerrero W, Odongo A, Varaine F. Impact of introducing human immunodeficiency virus testing, treatment, and care in a tuberculosis clinic in rural Kenya. Int J Tuberc Lung Dis 2010;14:611--5.
* Available at http://www.nltp.co.ke/docs/annual_report_2007.pdf.
† HIV testing in Kenya follows an established algorithm that involves parallel or serial testing with two rapid HIV tests. If the two tests have discordant results, a third confirmatory test (rapid test or other confirmatory test) is used as a tie-breaker. Rapid test results are provided to the patient on the same day that the test was conducted.
§ In Kenya, HIV-infected patients are eligible for ART if they have 1) a CD4 count of <200 cells/mm3, 2) a CD4 count of 200--350 cells/mm3 and WHO stage III disease, or 3) WHO stage IV disease (regardless of CD4 count).
What is already known on this topic?
TB is the leading cause of mortality worldwide for persons living with HIV infection, and HIV prevalence among TB patients in sub-Saharan Africa is estimated to be as high as 80%--90%.
What is added by this report?
Data from Kenya indicate increases in HIV testing among TB patients from 60% in 2006 to 88% in 2009; cotrimoxazole prophylaxis for opportunistic infections was provided to 92% of HIV-infected TB patients in 2009, but only 34% received potentially life-saving therapy with antiretroviral drugs during TB treatment.
What are the implications for public health practice?
Efforts to reach HIV-infected TB patients through national TB programs can be successful, but TB/HIV collaborative efforts must be strengthened to increase use of antiretroviral therapy among these patients.
FIGURE. Prevalence of HIV infection among newly registered TB patients,* by province --- Kenya, 2009
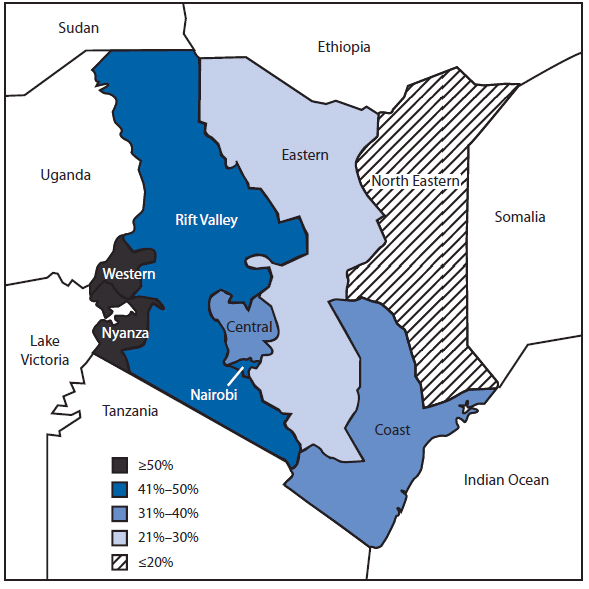
Abbreviations: HIV = human immunodeficiency virus; TB = tuberculosis.
* All patients who are currently diagnosed with active TB disease and are receiving TB treatment.
Alternate text: The figure above shows the prevalence of HIV infection among newly registered TB patients, by province in Kenya in 2009. The prevalence of HIV among TB patients varied widely by province, ranging from 5% in North Eastern Province to 70% in Nyanza Province.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All MMWR HTML versions of articles are electronic conversions from typeset documents.
This conversion might result in character translation or format errors in the HTML version.
Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr)
and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S.
Government Printing Office (GPO), Washington, DC 20402-9371;
telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to
mmwrq@cdc.gov.


