FIGURE 2. Recommended immunization schedule for persons aged 7 through 18 years --- United States, 2011 (for those who fall behind or start late, see the schedule below and the catch-up schedule [Table])
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail.
Errata: Vol. 60, No. 5
In "Recommended Immunization Schedules for Persons Aged 0 Through 18 Years --- United States, 2011," an error occurred on page 3, in Figure 2, "Recommended immunization schedule for persons aged 7 through 18 years --- United States, 2011." In that figure, the green bar indicating the range of recommended ages for catch-up immunization with the "MMR Series" should have extended across all three age ranges: 7--10 years, 11--12 years, and 13--18 years. The corrected figure is below.
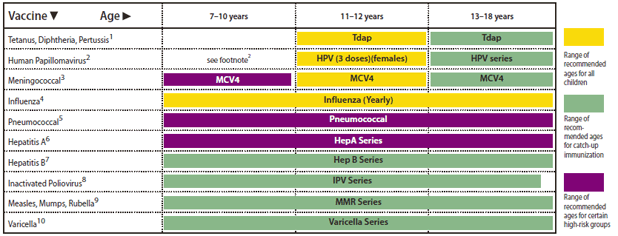
Alternate Text: The figure above shows the recommended immunization schedule for persons aged 7 through 18 years in the United States, 2011. A green bar indicating the range of recommended ages for catch-up immunization with the “MMR Series” should have extended across all three age ranges: 7–10 years, 11–12 years, and 13–18 years. The corrected figure is above.
This schedule includes recommendations in effect as of December 21, 2010. Any dose not administered at the recommended age should be administered at a subsequent visit, when indicated and feasible. The use of a combination vaccine generally is preferred over separate injections of its equivalent component vaccines. Considerations should include provider assessment, patient preference, and the potential for adverse events. Providers should consult the relevant Advisory Committee on Immunization Practices statement for detailed recommendations: http://www.cdc.gov/vaccines/pubs/acip-list.htm. Clinically significant adverse events that follow immunization should be reported to the Vaccine Adverse Event Reporting System (VAERS) at http://www.vaers.hhs.gov or by telephone, 800-822-7967.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All MMWR HTML versions of articles are electronic conversions from typeset documents.
This conversion might result in character translation or format errors in the HTML version.
Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr)
and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S.
Government Printing Office (GPO), Washington, DC 20402-9371;
telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to
mmwrq@cdc.gov.


