May 2022
June 19–23, 2022: Council of State and Territorial Epidemiologists (CSTE) Annual Conference, Louisville, Kentucky. Learn more
June 28, 2022, 3:00–4:00 PM ET: eSHARE special session: Modernizing the National Electronic Disease Surveillance System (NEDSS) Base System (NBS).
Update and reminder: processes for novel influenza A and influenza-associated pediatric mortality data
Jurisdictions should report data for the initial detection of novel influenza A virus infection and influenza-associated pediatric mortality to the CDC influenza program’s “Pediatric Death Reporting” web application available through the Secure Access Management Services (SAMS).
Jurisdictions should not report these data to the Message Validation, Processing, and Provisioning System (MVPS). Case notifications sent using HL7, NBS master message, or NETSS formats for these conditions will error out.
Instead, the CDC influenza program submits data on novel influenza A and influenza-associated pediatric mortality meeting NNDSS publication criteria to MVPS. The data are then published in the NNDSS tables on CDC WONDER.
Contact the CDC influenza program at fluviewsupport@cdc.gov for more information.
CDC held an eSHARE webinar, “Provide Your Jurisdiction’s Feedback: The Draft Hepatitis Message Mapping Guide v2.0,” on May 17, 2022. Visit the eSHARE archives to access the slides and recording.
On June 28, 2022, 3:00-4:00 PM ET, CDC will hold a special eSHARE session about CDC’s plans for modernizing NBS.
To join an eSHARE webinar, please see your calendar invitation or contact the CDC Electronic Data Exchange mailbox at edx@cdc.gov for login information with the subject line “eSHARE invitation.”
Are you new to working on case surveillance? Need help with learning acronyms?
Visit the NNDSS Technical Resource Center for definitions of common case surveillance acronyms.
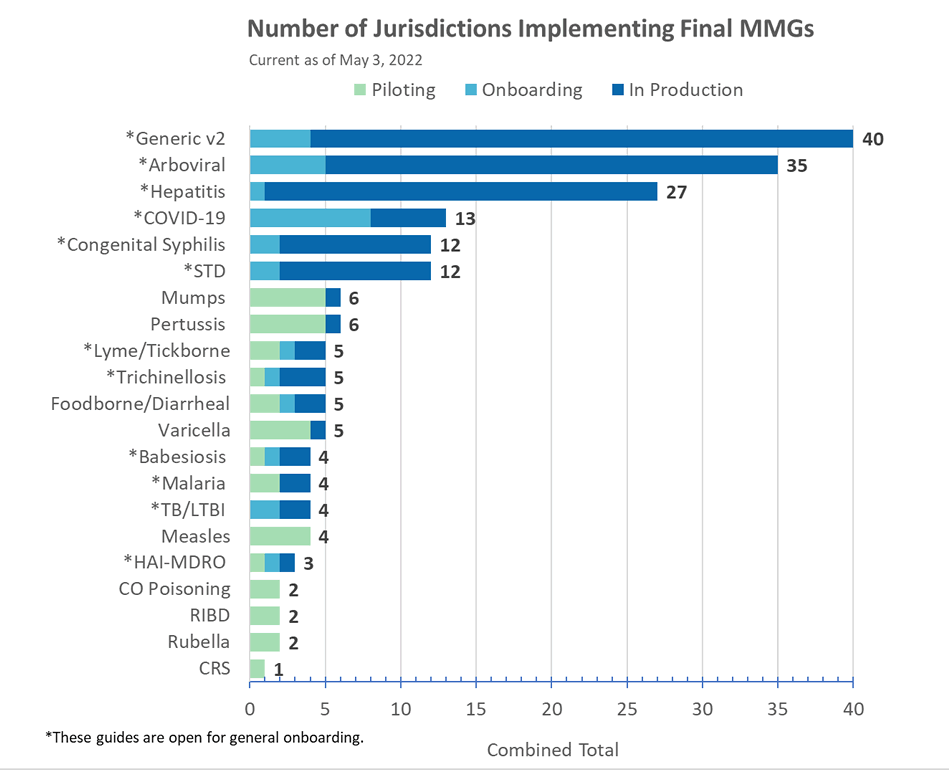
Carbon Monoxide Poisoning MMG
CDC launched the carbon monoxide poisoning MMG pilot in January 2022. The pilot cohort is currently completing gap analysis activities, with functionality testing on track to begin this month. CDC expects to finalize the guide in summer 2022.
COVID-19 MMG
Version 12 of the PHIN Vocabulary Access and Distribution System (VADS) COVID-19 Case Notification View is now available for COVID-19 MMG version 1.0 and version 1.1. The changes include:
- three additional values in Lab Test Type (COVID-19)
- one additional value for Vaccine Type (NND), and
- two additional values and six value name changes in Lab Test Interpretation (VPD)
Lyme and Tickborne Rickettsial Diseases (TBRD) MMG
Version 1.0.2 of the Lyme and TBRD MMG has now posted. This update includes formatting updates being applied across all guides. Additionally, version 3 of the PHIN VADS Lyme and TBRD Case Notification View is now available. The changes include:
- spelling correction of a value in Organism (Lyme) for Organism Name (LAB278) in Lyme,
- the most current Country (ISO) value set for Travel Country (82753-5) in TBRD, and
- 18 additional tests in Lab Test Type (TBRD) for Test Type (INV290)
STD and Congenital Syphilis (CS) MMGs
CDC posted updated STD (v1.2, v1.0.4) and CS (v1.1.2, v1.0.3) MMGs along with the associated implementation spreadsheets and PHIN VADS Case Notification Views to the NNDSS website.
The STD v1.2 MMG now contains a value set for the Type of Complications Indicator (INV920) that aligns with the updated case definition for identifying lymphogranuloma venereum. Tests have been added to Lab Test Type_STD and Lab Test Type_CS value sets for use in all versions of their respective guides.
Additionally, the guides include formatting updates being applied across all MMGs, including:
- updated MMG column description definition for “May Repeat” to provide additional information on “Y/#,”
- value set links changed from IDs of static versions of the value set at the time the MMG was finalized to object identifiers that bring the user to the current revision of the value set, and
- incorporation of GenV2.0.1 and updated test data into the test case scenario worksheet.
Please note the following:
- The STD v1.2 MMG uses the NEW PHIN VADS Case Notification View “STD Case Notification (v1.2)” Version 1.
- STD v1.0.x uses PHIN VADS Case Notification View “STD Case Notification” Version 13.
- The CS 1.2 MMG uses the NEW PHIN VADS Case Notification View “CS Case Notification (v1.1)” Version 1.
- CS v1.0.x uses PHIN VADS Case Notification View “CS Case Notification” Version 10.
If you are interested in joining a cohort or onboarding an MMG, please email the CDC Electronic Data Exchange mailbox at edx@cdc.gov.
Congratulations to the following jurisdictions in production as of May 2, 2022!
- Alaska for COVID-19 MMG
- Michigan for FDD MMG
- Kentucky for Hepatitis MMG
- Indiana for STD/CS MMGs
United States Digital Service begins case surveillance discovery sprint
As you may have heard, a team led by the United States Digital Service (USDS) started a six-week discovery sprint on Monday, May 9, 2022. CDC is collaborating with USDS to help identify opportunities to modernize the flow of case surveillance data.
USDS works on technology improvement projects impacting people served by the United States government. Since 2014, USDS has partnered with dozens of federal and state agencies to build more than 160 successful digital products. Learn more about how USDS conducts discovery sprints.
The sprint team will reach out to case surveillance experts in selected CDC programs and jurisdictions for time-limited participation in interviews and information gathering. The team began their interviews at CDC the week of May 9th and will begin reaching out to selected jurisdictions in the coming weeks.
Focus of the sprint
The sprint will examine the flow of case reporting and notification. The sprint team will review existing recommendations and available data, observe processes, and meet with CDC staff as well as state, territorial, local, and tribal (STLT) public health partners to gain insights and inform action steps.
The sprint will specifically look at the lifecycle of the data, starting at the point of care and ending in public health action. It will deepen our understanding of the current technologies, processes, and policies around disease surveillance data that will contribute to informing what a modernized, sustainable system could look like.
Building on successes
This sprint will build upon the successes of other previous and ongoing case surveillance improvement efforts. It aims to identify opportunities for reducing the burden and increasing the benefits throughout the entire case reporting and notification process at the STLT and national levels.
This effort is also aligned with CDC’s Data Modernization Initiative (DMI) and will address pre-existing challenges and current opportunities for modernizing the way we work together, and the systems, policies, and processes through which we collect and share public health data.
If you have any questions about the sprint, please contact Dr. Jennifer Layden, CDC Associate Deputy Director for Public Health Science and Surveillance; qbg5@cdc.gov, and DMI@cdc.gov. Please copy Zuzana Love, USDS Sprint Team; spy3@cdc.gov, on your e-mail.