Solving Public Health Problems
Yellow Fever (YF)
We’d like to collaborate with you! You can support public health by depositing your isolates in CDC’s Arbovirus Reference Collection (ARC). The ARC offers long-term curation, maintenance, and distribution of valuable isolates. Contact the ARC staff about depositing your isolates for long-term curation, maintenance, and distribution.
- Questions about ARC? Send an email to: reagents2@cdc.gov
- Other questions? Contact CDC-INFO.
- Ready to submit an isolate? Complete the ARC Submission Form [PDF – 3 pages] and the Simple Letter Agreement [PDF – 5 pages]. Then email the form and the letter to reagents2@cdc.gov.
If you need reagents, visit our Arboviral Reagent Ordering System.
Even with an effective vaccine, YF continues to be a public health problem:
- International exportation of YF occurred during the 2016 YF outbreaks in Angola and Democratic Republic of the Congo.
- Limited YF global vaccine supply
YF Public Health Problem #1
- Address the need for a readily available, standardized, YF serological testing kit that can be used in resource-limited countries
- Address the need for rapid YF diagnosis to quickly and effectively mobilize YF vaccine stocks in the event of an outbreak
YF MAC-HD kit
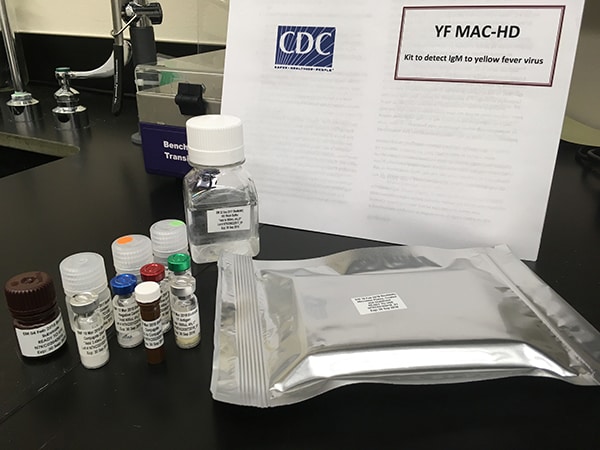
Easy-to-use Yellow Fever Virus MAC-HD Kit. Credit: CDC
Developed by Ms. Jane Basile at CDC1
Easy-to-use YFV MAC-HD kit
- Tests for IgM antibodies to YF
- Includes pre-measured reagents and ready-to-use plates
- Allows for assay completion in 3.5 hours
- Tests 8 or 24 serum samples per plate
- Kit (see photo) used by African and South American regional reference and national laboratories where YF is present.
Important benefits to public health
- Kit allows for rapid (half-day) YF serological diagnosis, critical during a YF outbreak to quickly and effectively mobilize vaccine stocks
- Kit format allows for standardization of YF serological testing within and across laboratories performing the test
ARC contribution
- Inactivated YF antigen
- Normal antigen
- YF positive control
- Conjugate
YF Public Health Problem #2
Address the need to differentiate between individuals infected with wild-type YF and individuals experiencing YF vaccination adverse events during YF outbreak vaccination campaigns
- Wild-type YF infection and YF vaccination adverse events are clinically and serologically indistinguishable.
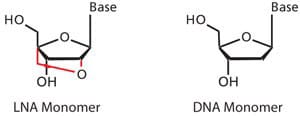
The YF 17D-specific real-time PCR assay uses Locked-nucleic acid (LNATM) bases to distinguish single nucleotide difference
YF 17D real-time PCR assay
Developed by Dr. Holly Hughes at CDC2
YF 17D-specific real-time PCR assay
- Distinguishes between wild-type YF and 17D (vaccine) strains
- Wild-type and 17D YF strains are genetically different by <10 nucleotides
- Uses Locked-nucleic acid (LNA™) bases capable of distinguishing single nucleotide differences
- Highly specific
- Incorporated into probe
Important benefits to public health
Differentiation between wild-type YF infection and YF vaccination adverse events during a YF outbreak aids in:
- Determining the nature and extent of the outbreak
- Decision-making by clinicians and public health officials
- Monitoring people during outbreak vaccination campaigns for vaccination adverse events
ARC contribution
- 17 different isolates of YF virus
- YF positive RNA controls
1 Publication: Development and validation of an ELISA kit (YF MAC-HD) to detect IgM to yellow fever virus. Basile AJ, Goodman C, Horiuchi K, Laven J, Panella AJ, Kosoy O, Lanciotti RS, Johnson BW. 2015. J Virol Methods, 225: 41-8.
2 Publication: Development of a Real-Time Reverse Transcription-PCR Assay for Global Differentiation of Yellow Fever Virus Vaccine-Related Adverse Events from Natural Infections. Hughes HR, Russell BJ, Mossel EC, Kayiwa J, Lutwama J, Lambert AJ. 2018. J Clin Micro, 56(6): e00323-18.