Benefits of Contributing to the Arbovirus Reference Collection (ARC)
We’d like to collaborate with you! You can support public health by depositing your isolates in CDC’s Arbovirus Reference Collection (ARC). The ARC offers long-term curation, maintenance, and distribution of valuable isolates. Contact the ARC staff about depositing your isolates for long-term curation, maintenance, and distribution.
- Questions about ARC? Send an email to: reagents2@cdc.gov
- Other questions? Contact CDC-INFO.
- Ready to submit an isolate? Complete the ARC Submission Form [PDF – 3 pages] and the Simple Letter Agreement [PDF – 5 pages]. Then email the form and the letter to reagents2@cdc.gov.
If you need reagents, visit our Arboviral Reagent Ordering System.
What Sets the ARC Apart from Other Repositories
- As a public health institution, we share materials as well as develop and share new diagnostic assays. Isolates in the ARC are used to:
- Develop antigens and antibody for serological assays
- Validate molecular assays
- Produce RNA controls for our molecular assays
- The public health community can access these assays through publications, distribution of protocols, and/or distribution of reagents.
- In addition, we work closely with the World Health Organization (WHO) as a WHO Collaborating Centre for Arthropod-Borne Viruses Reference and Research to support yellow fever and other arbovirus diagnostics around the world.
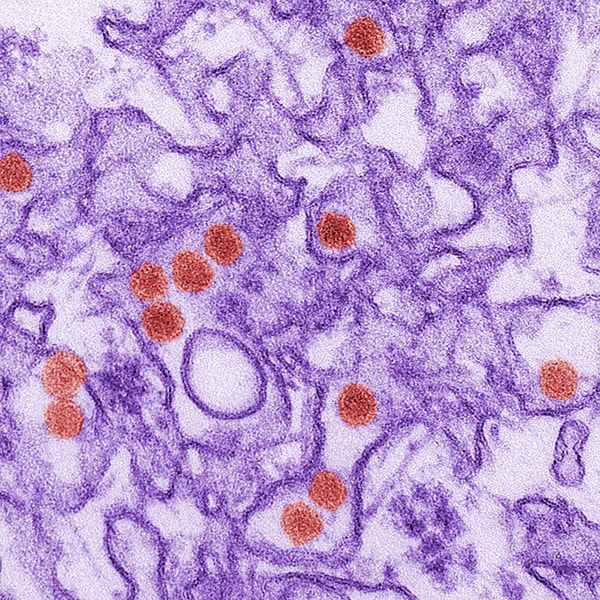
Digitally colorized transmission electron microscopic image of Zika virus, which is a member of the family, Flaviviridae. Virus particles, here colored red, are 40nm in diameter, with an outer envelope, and an inner dense core. Credit: CDC, Cynthia S. Goldsmith
Quality Control (QC) Program
- QC of viruses
- QC of cells used for replication of viruses
- QC of media used for virus replication and maintenance of cells
Centralized Distribution
- Individual researcher can refer requests to a repository.
- With a uniform process, individual researchers easily know where to obtain isolates.
- Repositories will have established distribution policies and procedures, including established transfer agreements.
Data Maintenance
Long-term sustainable programs, like the ARC, are best suited for maintenance of isolates and data. We’ve consistently seen data lost as researchers retire. By transferring data and isolates to the ARC, long-term management can be maintained.
- Our oldest isolate dates back to 1925.
- This highlights the long-term value of these isolates to the scientific community and the importance of the data associated with each isolate.
- Lab books and records are lost over time. Electronic, backed-up databases are necessary for long-term maintenance of data.
Cost
- The ARC pays for repository maintenance and meets high standards, making it ideal for long-term maintenance of historical isolates.
- The value of your isolates extends beyond your institution or career. The greater scientific community will thank you for your contribution.
How ARC is Different from Other Repositories
This negative-stained transmission electron microscopic image shows La Crosse (LAC) encephalitis virus ribonucleoprotein particles (RNP). Credit: CDC, Dr. J. Obijeski
ARC Product Insert
ARC product insert gives you all the information we know about the virus. We aim for complete transparency. Product inserts include:
- The complete history regarding the hands the virus passed through to get to us; including who isolated it and who submitted it to us, if these records are available.
- All QC testing results – all viruses are subjected to QC testing before being flagged “ready to transfer.” This may cause a delay in your request if it’s a virus that requires QC testing.
- ARC staff record notes on all replication methods used when working with the virus. This includes notes about plaque morphology, what cell types work best for virus replication, etc.
- Any irregularities or discrepancies noted in the literature regarding the isolate. For example, if the virus or isolate has been misspelled or referred to differently at different times throughout its history.
- If the virus has ever gone through any plaque purification and the reason for the plaque purification.
Transfer Agreements
- Along with CDC’s technology transfer office, we have developed a simple letter agreement for use with most transfers.
- This prevents the recipient from performing third-party transfers.
- All virus transfers from the ARC require a signed agreement.
- When you deposit with us, we can negotiate what types of transfers are acceptable. We prefer transfers to non-profit entities without limitations. Requests from for-profit entities can be referenced back to the depositor.
- The ARC database allows us to place distribution restrictions on the individual isolates and track depositor information.
Reagent Production
- To support the arbovirus public health community, the ARC produces non-infectious antigen, antibody, and RNA that can be used in CDC assays or other assays.
Protocol Development and Distribution
- Distribute protocols for diagnostic assays
- Distribute protocols for reagent production
- Distribute protocols for virus replication and maintenance