Key points
- Follow standard precautions, including wearing appropriate PPE, when collecting specimens.
- Skin lesion material is the specimen type recommended for monkeypox diagnostic testing.
- Use only sterile, synthetic swabs with plastic, wood, or thin aluminum shafts to collect specimens. Do NOT use sharps or unroof lesions.
- Specimen collection, storage, and shipping of human specimens are subject to Clinical Laboratory Improvement Amendments (CLIA) restrictions.
General guidance
Review Biosafety Laboratory Guidance for Handling and Processing Monkeypox Specimens for recommended laboratory procedures and biosafety guidelines when collecting, handling, and processing specimens.
Specimen collection, storage, and shipping of human specimens are subject to Clinical Laboratory Improvement Amendments (CLIA) restrictions. Therefore, CDC recommends contacting the testing laboratory testing facility to determine their specific requirements for monkeypox virus (MPXV)testing.
Collection
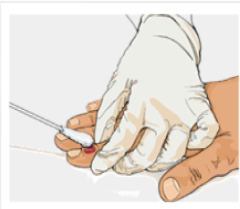
Wear all recommended PPE when collecting a specimen from a person with suspected or confirmed monkeypox.
- Only sterile, synthetic swabs (including but not limited to polyester, nylon, or Dacron) with plastic, wood, or thin aluminum (wire) shafts should be used to collect suspected or confirmed monkeypox specimens for diagnostic testing.
- Do not use cotton swabs or swabs that have foam or other material placed at the bottom.
- Skin lesion material, including swabs of lesion surface, exudate, or lesion crusts are the specimen types recommended for laboratory testing MPXV specimens.
- Procedures and materials used for collecting specimens may vary depending on the phase of the rash (e.g., swabs from lesion surface or crust from healing lesion).
- Do not unroof or aspirate of lesions or otherwise use sharp instruments for monkeypox testing due to the risk for sharps injury.
Lesion material collection
- Collect two swabs from each lesion, preferably from different locations on the body or from lesions that differ in appearance (e.g., a pair of swabs for each lesion with a total of 2-3 lesions).
- Vigorously swab each lesion, avoiding contamination of gloved hands, to ensure adequate viral DNA is collected.
- Place swabs from lesions, crusts, and exudate in separate tubes.
- Insert each swab into a sterile container such as a sterile tube or urine container. Glass containers are not recommended.
- Carefully bend to break the swab's shaft to fit inside the sterile container (or if it fits, place the entire swab into the container).
- After completely securing the lid, wipe the container with an EPA-approved disinfectant for emerging viral pathogens.
- CDC recommends placing parafilm around the lid of the container for additional leak-proof protection, but it's not required.
- After handling the swab and container, remove gloves, wash your hands (hand hygiene), and don a new pair of gloves.
Specimen labeling
Clinical Laboratory Improvement Amendments (CLIA) require laboratories to ensure positive specimen identification and optimum integrity of a patient's specimen using 2 identifiers and the specimen information.
Clearly label the specimen container with the patient identifiers and include the appropriate specimen information prior to collecting the specimen. Identifiers must be visible, and labels cannot cover the identifiers. Patient identifiers should include at least 2 of the following:
- Patient name (full first and last name, no initials)
- Patient date of birth (MM/DD/YYYY)
- Patient sex assigned at birth
- A unique ID generated at the time of collection (e.g., a medical record number). A State Public Health Lab ID does not satisfy the requirement
Specimen information should include but is not limited to:
- Collection site (e.g., left arm, upper left groin, right cheek, etc.)
- Collection date
- Specimen type
Contact the laboratory facility if additional information is required on the label.
Storage
- After specimen collection, store specimens in sterile leak-proof containers.
- Specimens do not have to be transported in 2 mL O-ring tubes if the original container is sterile and leak-proof.
- Use a durable container for the required shipping and temperature conditions.
- Glass containers are not recommended.
- Use parafilm for specimens in transport media as an extra safety precaution.
Contact the appropriate laboratory facility to confirm specimen storage prior to shipment.
Shipment
Clade I MPXV is a select agent. If the clade is undetermined or clade I MPXV is identified in a clinical or diagnostic specimen, select agent and toxin regulations apply; however, an exemption may apply.
If non-variola orthopoxvirus or clade II MPXV is identified in a clinical or diagnostic specimen, the material is not a select agent and the select agent and toxin regulations do not apply.
Ship specimens and material suspected or confirmed to contain clade II MPXV as UN 3373 Biological Substance, Category B.
- When preparing specimens for shipment, consider individually bagging specimens in transport media so that if a leak does occur, it does not cause the rejection of all specimens.
- Specimens received outside of acceptable temperature ranges may be rejected. Contact the appropriate laboratory facility to determine shipping recommendations. Shipping on dry ice is often recommended.
The primary receptacle and secondary packaging should maintain their integrity at the temperature of the refrigerant used, even if the refrigerant's temperature changes. Packages containing dry ice should be designed to prevent pressure buildup, package rupture, and allow gas release. Personnel trained per U.S Department of Transportation (DOT) hazmat transportation training requirements should pack and ship specimens.
Ensure the outer package is appropriately marked and labeled as follows:
- Sender's name and address
- Recipient's name and address
- Biological substance label
- Proper shipping name, Biological substance, Category B
- UN identification number, UN 3373
- Name and telephone number of the person responsible for shipment (available during regular business hours; optional if the information is on the airway bill)
- Shipper's Declaration is not required for UN 3373 Biological Substances Category B shipped specimens
- If an Air Waybill is used, the "Nature and Quantity of Goods" box should show "UN 3373 Biological Substance, Category B" and the number of packages
- Class 9 label, including UN 1845, and net weight, if packaged with dry ice and identified as Carbon Dioxide, solid, or Dry ice
Shipping specimens to CDC
In most situations, specimens should be sent to the appropriate state public health laboratory (SPHL) or a commercial laboratory for initial testing.
If authorized to send directly to CDC for testing, each specimen must be accompanied by a CDC 50.34 form or entered in the CDC Specimen Test Order and Reporting Web Portal (CSTOR). If neither of these is an option or your laboratory is submitting a high volume of specimens, the global file accessioning template (GFAT) is available. Contact poxviruslab@cdc.gov to receive this form.
