CDC Experts Help India Scale-up Laboratory Systems in Response to COVID-19’s Second Wave
February 15, 2022

In April 2020, CDC staff offer training on how to make alcohol-based hand rub at the All India Institute of Medical Sciences during the COVID-19 pandemic. Photo: CDC India office
On January 30, 2020, India, with a population of 1.39 billion, reported its first case of COVID-19. The CDC office in India (CDC India), with its 40 U.S. assignees and locally employed staff, quickly activated its incident management system to support both the U.S. Mission and the Government of India (GOI) with COVID-19 response efforts. To ensure effective management and rapid deployment of CDC India staff, teams were formed and aligned with the most critical response activities. They regularly provided situational reports to U.S. leadership and other sections within the U.S. Mission in India. CDC India was able to leverage decades of cooperation with the GOI on global health security efforts to build laboratory capabilities and provide technical assistance and support in key areas of the response.
By March 2020, the threat of COVID- 19 loomed large, and the GOI worked hard to establish an effective response plan. At that time, CDC India was already collaborating with the National Health System Resource Center (NHSRC), the technical wing of the GOI’s National Health Mission (NHM), to create a network of Integrated Public Health Laboratories (IPHL) for the COVID-19 response. Building on a collaboration that saw the development of India’s first model IPHL in the Raipur District in the state of Chhattisgarh, CDC India was able to support NHM in developing operational guidelines to establish an IPHL network across the country. The IPHL concept immediately found favor at the highest level of the GOI and was formally incorporated into India’s COVID-19 response strategy. The GOI announced a pandemic economic stimulus package of USD 266 billion to establish a network of IPHL in 730 districts across the country. This effort helped to strengthen and expand India’s core public health lab capabilities and build on existing investments to expand laboratory testing capacity.

CDC Lab Team in collaboration with U.S. Biothreat reduction program and NSF International, organized the second-ever biosafety cabinet certification training in Gurugram, 2021. Photo: CDC India office
Almost a year later (mid-March 2021), India faced a second wave of COVID-19 infections that strained the country’s public health system. Infection rates soared to about 32 million and more than 400,000 deaths. In response, CDC developed peer-to-peer relationships with India’s National Public Health Institute (known as “NCDC”, or the National Centre for Disease Control) and the GOI. In accordance with international standards, CDC India provided technical guidance and training on safe COVID-19 sample collection, testing, and transport to over 10,000 healthcare workers across 210 districts in the country. Participants included frontline workers of the GOI National Disaster Response Force and research scientists involved with COVID-19 biorepositories (facilities that collect, catalogue and store samples of biological material for laboratory research) in the Department of Biotechnology.
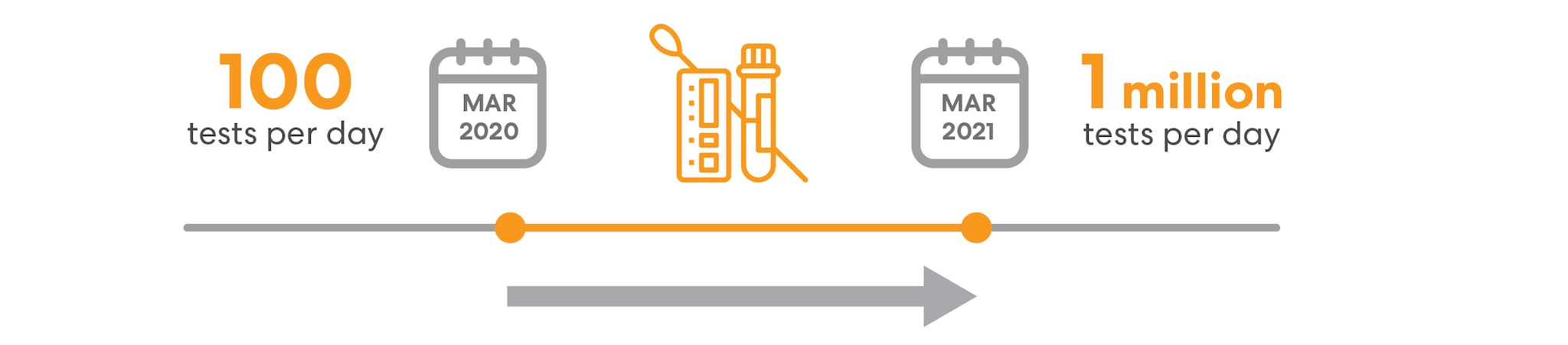
CDC experts also played a pivotal role in supporting the GOI scale-up of lab capabilities by participating in several national and sub-national advisory and expert committee groups. These interactions helped to shape the implementation of new GOI resources and programs. CDC’s technical expertise contributed to the rapid expansion of SARS-COV-2 testing in India from approximately 100 tests per day in March 2020 to around 1 million tests per day by the end of March 2021.
In addition, CDC’s Lab and Infection Prevention and Control teams jointly developed a guidance document on safe practices for COVID-19 testing laboratories, which was accepted by the All India Institute of Medical Sciences in New Delhi and is now available on its website. CDC also conducted online trainings on the basics of laboratory and field biosafety activities for GOI, academic partners, implementing partners, professional societies, and state health departments.

CDC India trains health workers on “Sample Collection, Packaging, and Safe Transportation along with Rapid Diagnostic Testing” as a part of outbreak preparedness training. Photo: CDC India office
CDC’s longstanding partnership with the GOI spans over 50 years and has strengthened during the COVID-19 pandemic. GOI’s efforts and investments in laboratories, along with CDC’s collaboration and technical support, have significantly improved and streamlined specimen transport systems and supply chains, and they have provided quality-assured results to inform public health action across the country and the region. GOI has included CDC’s model of IPHL in the new health plan announced by the Prime Minister of India and known as the Prime Minister’s Ayushman Bharat Health Infrastructure Mission. CDC’s support has strengthened workforce capacity by training thousands of postgraduate medical students and first-line responders on public health emergency management. Alumni of these programs are either leading or supporting GOI COVID-19 response efforts in various capacities.
Many of CDC India’s pre-pandemic collaborations, programs, and relationships with GOI laid a strong foundation for India’s ongoing response to COVID-19. India’s sizeable population, location, and disease burden have made the GOI an important CDC partner and helped to inform and advance global health security. This was aptly summarized by CDC India Country Director Dr. Meghna Desai, who said, “The past 18 months have been some of the most challenging and fulfilling times of my 20-year global health career. The peer-to-peer engagement that is the hallmark of CDC’s global health work was exactly the need of the hour for India.”