What to know
CDC's Clinical Standardization Programs aims to ensure that laboratory measurements for disease biomarkers used in patient care, research, and public health are accurate.

CDC Hormones Standardization Programs (HoSt)
Standardization of measurement procedures
CDC help laboratories with lab-developed tests and assay manufacturers with calibration. The HoSt program assesses the assay performance (bias and imprecision) of the lab or assay manufacturer throughout one year.
The program consists of two independent phases.
Schematic of the CDC Hormone Standardization Programs
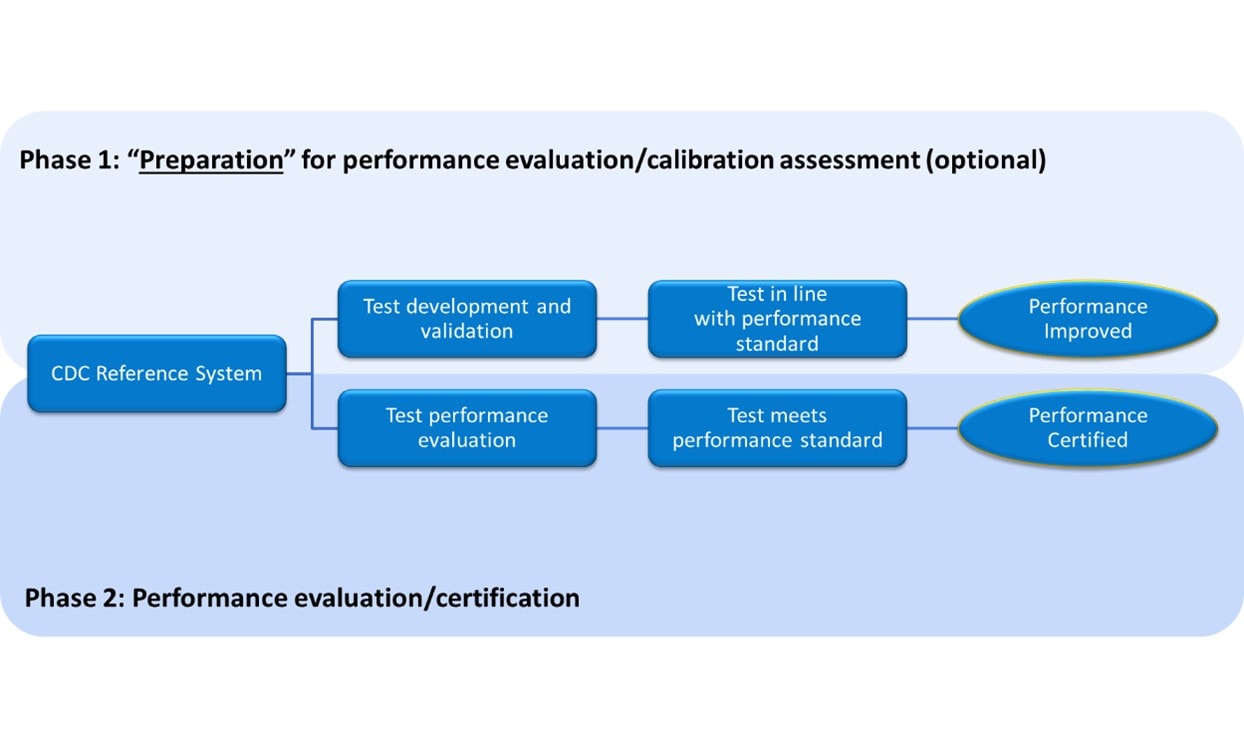
HoSt Phase 1- assessment and improvement of analytical performance
CDC provides individual donor samples with reference values, which participants use to assess and improve the analytical performance of their laboratory method. Typical parameters assessed with these samples are calibration bias, sample-specific bias (i.e., selectivity) and precision.
How this is done:
- Participants receive sets of serum samples (with reference values assignments) from individual donors containing varying concentrations of testosterone and estradiol assigned with the CDC reference methods. Typical sets contain 40 samples, however, this can be customized to include any number of samples up to 120, as needed by the participant. The serum samples are non-pooled, single donor sera prepared following CLSI protocol C37 (3) from male and female donors, unless otherwise specified by the participant.
- Participants measure these samples and compare their results with the reference values provided. This step assesses the analytical accuracy of their laboratory methods and identifies potential problems.
- Participants work to improve their measurement accuracy based on the finding of their comparison. CDC can provide assistance with this step, if requested.
To obtain Phase 1 samples for assessment of accuracy, contact Standardization@cdc.gov
HoSt Phase 2 – verification and certification of analytical performance
Participants can participate in this phase independent to Phase 1.
Phase 2 is the Standardization- Certification Phase. Laboratory tests, used for patient care and public health, must meet certain requirements for accuracy and precision. With the Hormone Standardization Certification Program, CDC evaluates whether laboratory tests for testosterone and estradiol meet certain analytical performance criteria. This phase can also be used to verify metrological traceability as described in ISO standard 17511:2020.
CDC provides individual donor samples without reference values. Participants measure the steroid hormones in these samples and report results to CDC, which compares results to actual reference values and evaluates the analytical performance such as measurement bias and imprecision. Participants meeting analytical performance criteria receive a certificate.
How this is done:
- CDC provides participants with 10 blinded serum samples every quarter. Participants are unaware of the testosterone and estradiol concentrations in these samples.
- Participants follow a specific protocol to analyze samples, and report results back to CDC.
- CDC takes participants' data, reported from four consecutive quarters, and compares it to the actual concentration determined with CDC's reference method.
- CDC evaluates the accuracy and precision of participants' data. Values reported from participants on these serum samples are used for bias assessment as described in Clinical Laboratory and Standards Institute guidelines EP9-A2 (4) "Method Comparison and Bias Estimation using Patient Samples."
- Participants receive a comprehensive report about the assessment. Laboratory tests that meet bias and precision analytical performance criteria receive a certificate and are listed on the HoSt list of certified labs for testosterone or estradiol. Current acceptable criteria are derived from data on published biological variability.
CDC provides preliminary reports for each quarterly challenges and technical assistance to resolve potential problems, thus ensuring the participant's long-term success in maintaining standardized measurements.
Certificates are valid for one year and can be renewed by re-enrolling in the program. Enrollments can be done at any time throughout the year. To enroll in the program, contact Standardization@cdc.gov.
All participants remain anonymous. Listing of successful participation on the CDC Website is voluntary.
Current analytical performance criteria for certification
| Analyte | Accuracy | Precision |
|---|---|---|
| Testosterone1 | ±6.4% mean bias | <5.3%** |
| Estradiol2 | ±12.5% bias if >20 pg/mL, ±2.5 pg/mL bias if ≤20 pg/mL |
<11.4%** |
** criteria included in performance report but not currently used for certification
Validation/certification protocols
The CDC HoSt Testosterone program started in January 2010.
- HoSt – Standardization of Serum Total Testosterone Measurement Protocol – Updated April 2023
- HoSt Testosterone Certified Procedures – Updated November 2023
Phase 2 of the CDC HoSt Estradiol (E2) program started in 2014.
- HoSt – Standardization of Serum Total Estradiol Measurement Protocol – Updated April 2023
- HoSt Estradiol Certified Procedures – Updated November 2023
