Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail.
Antibodies Cross-Reactive to Influenza A (H3N2) Variant Virus and Impact of 2010–11 Seasonal Influenza Vaccine on Cross-Reactive Antibodies — United States
Since August 2011, a total of 12 human infections with influenza A (H3N2) variant viruses with genes from avian, swine, and human viruses (i.e., A [H3N2]v) that had acquired the M gene from influenza A (H1N1)pdm09 virus have been reported to CDC. Eleven of the cases occurred in children aged <10 years. In six cases, no history of recent exposure to swine was noted, suggesting that human-to-human transmission had occurred (1–3). This new gene constellation for A (H3N2)v viruses and its temporal association with an increase in human cases of A (H3N2)v highlight the need to better understand the risk for human infection with these viruses and the extent to which current seasonal vaccines might elicit cross-reactive antibodies to them. CDC conducted a preliminary analysis to evaluate the age-specific presence of serum cross-reactive antibody in U.S. populations vaccinated or not vaccinated with the 2010–11 seasonal trivalent influenza vaccine (TIV). The results indicated that 1) little or no cross-reactive antibody to A (H3N2)v exists among children aged <10 years, 2) immunization with the 2010–11 TIV had no impact on cross-reactive antibody levels in those aged <3 years, 3) cross-reactive antibody was detected in 20%–30% of those aged ≥10 years, and 4) among adults, vaccination with TIV provided a modest boost to the level of cross-reactive A (H3N2)v antibodies. Receipt of seasonal influenza vaccine continues to be recommended to protect against circulating human influenza viruses for all age groups and might provide limited protection against A (H3N2)v infection in the adult population. A vaccine virus specific for A (H3N2)v has been developed and could be used to produce an H3N2v vaccine, if needed.
Serum samples tested in this study were from two sources, a 2010–11 TIV study and the 2007–2008 National Health and Nutrition Examination Survey (NHANES). The TIV study sera included serum samples from children aged 6–35 months, adults aged 18–49 years, and older adults aged ≥65 years, all collected in the fall of 2010 before vaccination and again 3–4 weeks after vaccination. The children had no history of influenza vaccination and received 2 doses of vaccine, 4 weeks apart, with the postvaccination serum sample collected 3–4 weeks after the second dose. The TIV study serum samples were acquired through a contract and received as anonymous samples and thus were exempt from CDC institutional review board review. NHANES was the source of samples from children aged 4–17 years, which were part of a larger set received by CDC labeled only with age and date of sample collection. The protocol was reviewed and approved by the institutional review board of CDC's National Center for Immunization and Respiratory Diseases and the Research Ethics Review Board of CDC's National Center for Health Statistics.
Hemagglutination inhibition (HI) and microneutralization (MN) assays were performed following standard procedures* using A/Minnesota/11/2010 (H3N2)v and seasonal influenza viruses, A/Wisconsin/67/2005 (H3N2), and A/Perth/16/2009 (H3N2). A/Minnesota/11/2010 (H3N2)v is antigenically and genetically closely related to A (H3N2)v isolated from humans in 2011 (3,4). MN assays quantify antibodies that neutralize and prevent infection, whereas HI assays detect antibodies that inhibit the binding of virus to receptors on red blood cells. Serum HI titers of ≥40 are associated with reduction in the risk for influenza infection in adult populations. Although the 50% protective titer for the MN assay is not known, a previous study of antibody responses to persons infected with influenza A (H1N1)pdm09 virus showed that the MN titer was generally twofold higher than the HI titer when the HI titer was ≤160 (5). For this reason, titer achievements of ≥80 for the MN assay are presented.
Among 20 children aged 6–35 months, no evidence was found of antibodies to A (H3N2)v either before or after vaccination with the 2010–11 TIV, whereas 40% or 45% of children demonstrated seroconversion (i.e., a fourfold or greater increase in antibody titer) to the seasonal A (H3N2) virus contained in the vaccine by HI or MN assays, respectively, and 40% of children achieved HI titers of ≥40 and MN titers of ≥80 (Table 1). In contrast, among 30 adults aged 18–49 years, somewhat higher levels of prevaccination antibody to A (H3N2)v were detected, with 33% of this age group achieving HI titers of ≥40 and 43% achieving MN titers of ≥80. The proportion of adults aged 18–49 years with cross-reactive HI and MN antibody to A (H3N2)v increased to 50% and 63%, respectively, after immunization with TIV. As expected, after vaccination, 80% of these adults achieved HI titers of ≥40, and 70% achieved MN titers of ≥80 to the seasonal A (H3N2) vaccine component. Adults aged ≥65 years also exhibited prevaccination antibody to A (H3N2)v, with 17% of 30 achieving HI titers of ≥40 and 30% achieving MN titers ≥80. This increased to 40% postvaccination by either assay. By comparison, 67% and 90% of adults aged ≥65 years exhibited postvaccination HI titers ≥40 or MN titers ≥80, respectively, to the seasonal A (H3N2) vaccine component. Therefore, in these two adult populations, receipt of TIV boosted the levels of antibodies to A (H3N2)v, but to a lesser extent than the antibody response to the A (H3N2) vaccine component.
Because the TIV study did not include persons aged 4–17 years, single serum samples collected during 2007–2008 as part of NHANES were used to assess the level of cross-reactive antibody to A (H3N2)v in this age group. The NHANES samples were stratified into two age groups (4–9 years and 10–17 years) based on analyses of HI and MN titers with A (H3N2)v viruses that showed a statistical difference between them by either assay (Table 2). Among 38 children aged 4–9 years, cross-reactive geometric mean HI and MN antibody titers to A (H3N2)v essentially were at baseline, with only 5% or 8% of children exhibiting HI titers ≥40 or MN titers ≥80 to H3N2v, respectively. Among 34 youths aged 10–17 years, a higher level of cross-reactive antibody to A (H3N2)v was detected. Among these older children, 26% and 29% had HI titers ≥40 or MN titers ≥80 to A (H3N2)v, respectively. For both age groups, HI titers ≥40 to a seasonal A (H3N2) virus that circulated in the years just before sample collection were detected in approximately two thirds of children.
Approximately one third of persons tested aged 10–49 years had cross-reactive antibodies that might provide some protection from infection with contemporary A (H3N2)v viruses (Figure). A slight drop in the cross-reactive antibody rates in persons aged ≥65 years was observed, but only the decrease in MN geometric mean titer was statistically significant (p=0.05). Children aged <10 years had minimal cross-reactive antibodies, suggesting that they are at higher risk for infection with A (H3N2)v viruses.
Reported by
Alicia Branch, PhD, Vic Veguilla, MPH, Eric Gillis, MS, Carrie Reed, MD, Heather Noland, Leilani Thomas, Peter Browning, Amanda Balish, MS, Alicia Fry, MD, Nancy Cox, PhD, Jacqueline M. Katz, PhD, Kathy Hancock, PhD, Influenza Div, National Center for Immunization and Respiratory Diseases, CDC. Corresponding contributor: Kathy Hancock, khancock@cdc.gov, 404-639-5449.
Editorial Note
Human infections with influenza A (H3N2)v were reported with increased frequency in 2011 compared with previous years; enhanced surveillance might be a contributing factor. The results in this report suggest that children aged <10 years have very low or undetectable levels of HI and neutralizing antibodies that react with A (H3N2)v and are likely to be the population most susceptible to infection with A (H3N2)v viruses among groups studied. These data are consistent with the findings that 11 of the 12 influenza A (H3N2)v cases reported in 2011 were in children aged <10 years (2).
This study also found that some persons aged ≥10 years had antibodies that are cross-reactive with A (H3N2)v. Twenty-six percent of those aged 10–17 years, 33% of those aged 18–49 years, and 17% of those aged ≥65 years had an HI titer ≥40 to A (H3N2)v, which is generally accepted to represent a 50% protective titer for seasonal influenza viruses in adult populations (6). These antibody levels suggest that persons aged ≥10 years might be less susceptible to infection with A (H3N2)v viruses, although the relationship between cross-reactive antibodies and cross-protective antibodies in A (H3N2)v infections has not been determined. Furthermore, a recent study suggests that the titer that is 50% protective might be higher for children (7). These levels of cross-reactive antibodies in unexposed populations are higher than those that were observed in older children and adults to influenza A (H1N1)pdm09 before the 2009 pandemic and are more similar to those detected in older adults at that time (8,9).
Vaccination of adults (aged 18–49 years) and older adults (aged ≥65 years) boosted the levels of cross-reactive antibodies to A (H3N2)v, but to a lesser extent than the response to the A (H3N2) component of the vaccine. In children aged <3 years, receipt of TIV did not result in an antibody response to A (H3N2)v. A serologic study in Canada also showed no evidence of cross-reactive antibodies in children aged <10 years, and receipt of influenza vaccine did not induce a cross-reactive antibody response in those aged ≤4 years (10).
The findings in this report are subject to at least four limitations. First, the number of subjects in each age group was small, and the serum samples collected during 2007–2008 might underestimate or overestimate the current levels of cross-reactive antibodies in persons aged 4–17 years. Testing of a larger number of serum samples collected more recently is under way. Second, because of the participant selection criteria for the pediatric population, this population might not be representative of all children aged 6–35 months. Third, the populations aged 4–9 years and 10–17 years described in this report were not part of a vaccine study, so the impact of immunization with TIV in these age groups was not determined. Finally, antibody responses to viral antigens other than hemagglutinin and T-cell responses were not assessed but also might contribute to immunity to A (H3N2)v.
The composition of the 2011–12 seasonal TIV is identical to the 2010–11 vaccine evaluated in this report and is expected to provide limited cross-protection from A (H3N2)v in adults and no cross-protection in young children. In the event of sustained human-to-human transmission of (H3N2)v, an A (H3N2)v–specific vaccine would provide optimal protection for all ages. An A (H3N2)v reassortant vaccine strain based on the A/Minnesota/11/2010 virus has been developed and could be used to produce an H3N2v vaccine, if needed (3). Updated information and guidance documents related to A (H3N2)v viruses are available online from CDC at http://www.cdc.gov/flu/swineflu/influenza-variant-viruses.htm.
References
- CDC. Limited human-to-human transmission of novel influenza A (H3N2) virus—Iowa, November 2011. MMWR 2011;60:1615–7.
- CDC. Update: influenza A (H3N2)v transmission and guidelines—five states, 2011. MMWR 2012;60:1741–4.
- Lindstrom S, Garten R, Balish A, et al. Human infection with novel reassortant influenza A (H3N2)v viruses, United States, 2011. Emerg Infect Dis 2012;18 (in press).
- Shu B, Garten R, Emery S, et al. Genetic analysis and antigenic characterization of swine origin influenza viruses isolated from humans in the United States, 1990–2010. Virology 2012;422:151–60.
- Veguilla V, Hancock K, Schiffer J, et al. Sensitivity and specificity of serologic assays for detection of human infection with 2009 pandemic H1N1 virus in U.S. populations. J Clin Microbiol 2011;49:2210–5.
- Katz JM, Hancock K, Xu X. Serologic assays for influenza surveillance, diagnosis and vaccine evaluation. Expert review of anti-infective therapy. Expert Rev Anti Infect Ther 2011;9:669–83.
- Black S, Nicolay U, Vesikari T, et al. Hemagglutination inhibition antibody titers as a correlate of protection for inactivated influenza vaccines in children. Pediatr Infect Dis J 2011;30:1081–5.
- Hancock K, Veguilla V, Lu X, et al. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N Engl J Med 2009;361:1945–52.
- Reed C, Katz JM, Hancock K, Balish A, Fry AM. Prevalence of seropositivity to 2009 pandemic influenza A/H1N1 virus in the United States by December 2009. Presented at the Council of State and Territorial Epidemiologists (CSTE) Annual Conference, Pittsburgh, PA, June 2011.
- Skowronski DM, De Serres G, Janjua NZ, et al. Cross-reactive antibody to swine influenza A(H3N2) subtype virus in children and adults before and after immunisation with 2010/11 trivalent inactivated influenza vaccine in Canada, August to November 2010. Eurosurveillance 2012;17(4).
* Additional information available at http://www.who.int/influenza/resources/documents/manual_diagnosis_surveillance_influenza/en/index.html.
What is already known on this topic?
Twelve human infections with influenza A (H3N2)v virus were detected in the United States in 2011, compared with eight cases in the preceding 2 years. Most of these cases were in children aged <10 years.
What is added by this report?
Children aged <10 years have few or no cross-reactive antibodies to A (H3N2)v virus, but some older children and adults do have cross-reactive antibodies to the virus. Vaccination with the 2010–11 seasonal influenza vaccine had no impact on cross-reactive antibody levels in children aged <3 years but did boost cross-reactive antibodies in adults aged 18–49 years and ≥65 years, but only to levels that were lower than to seasonal A (H3N2) virus.
What are the implications for public health practice?
In the event of sustained human-to-human transmission of A (H3N2)v virus, children aged <10 years are likely to be the most susceptible to infection among groups studied. Although unlikely to protect against A (H3N2)v in this susceptible age group, receipt of seasonal influenza vaccine continues to be recommended to protect against circulating human influenza viruses for all age groups and might provide some protection against A (H3N2)v infection in the adult population. A vaccine virus specific for A (H3N2)v has been developed and could be used to produce an H3N2v vaccine, if needed.
FIGURE. Percentage titer achievement for cross-reactive hemagglutination inhibition (HI) and microneutralization (MN) antibodies to influenza A (H3N2) variant virus* before and after receipt of 2010–11 trivalent inactivated seasonal influenza vaccine, by age group — United States
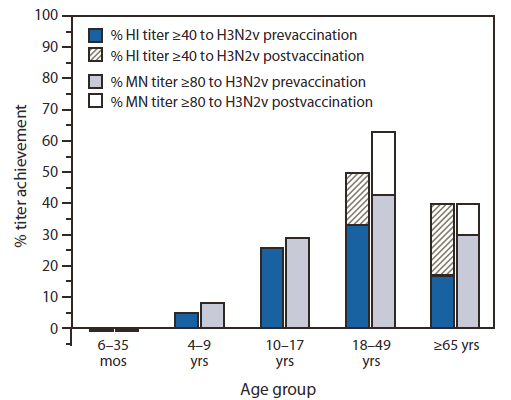
* A/Minnesota/11/2010.
Alternate Text: The figure above shows the percentage titer achievement for cross-reactive hemagglutination inhibition and microneutralization antibodies to influenza A (H3N2) variant virus before and after receipt of 2010-11 trivalent inactivated seasonal influenza vaccine, by age group in the United States. Approximately one third of persons tested aged 10-49 years had cross-reactive antibodies that might provide some protection from infection with contemporary A (H3N2)v viruses.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All MMWR HTML versions of articles are electronic conversions from typeset documents.
This conversion might result in character translation or format errors in the HTML version.
Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr)
and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S.
Government Printing Office (GPO), Washington, DC 20402-9371;
telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to
mmwrq@cdc.gov.


