Chapter 11: Pneumococcal
Manual for the Surveillance of Vaccine-Preventable Diseases
Authors: Ryan Gierke, MPH; Lesley McGee, PhD; Bernard Beall, PhD; Tamara Pilishivili, MPH
Disease Description
Streptococcus pneumoniae (pneumococcus) is a gram-positive bacterium with more than 90 known serotypes. Pneumococcus is spread by airborne droplets and is a leading cause of serious illness, including bacteremia, meningitis, and pneumonia among children and adults worldwide.[1,2] Although all serotypes can cause serious disease, a relatively limited number of serotypes cause the majority of invasive pneumococcal disease (IPD).
The Centers for Disease Control and Prevention’s (CDC’s) Active Bacterial Core Surveillance (ABCs) has tracked IPD in selected regions of the United States since 1994. ABCs data indicate that individuals <2 and ≥65 years of age have the highest rates of invasive disease (Table 1). [2,3] Approximately 10% of all patients with invasive pneumococcal disease die of their illness, but case-fatality rates are higher for the elderly and patients with certain underlying illnesses. [3,4]
Table 1. Incidence of pneumococcal infections in the United States[3]
| Age (years) | Disease Incidence Cases/100,000 (number of cases) | Death Rate Deaths/100,000 (number of deaths) |
|---|---|---|
| <1 | 17.7 (702) | 0.20 (8) |
| 1 | 12.6 (500) | 0.20 (8) |
| 2−4 | 5.07 (606) | 0.13 (16) |
| 5−17 | 1.23. (659) | 0.00 (0) |
| 18−34 | 2.33 (1,757) | 0.08 (60) |
| 35−49 | 6.48 (3,982) | 0.46 (284) |
| 50−64 | 14.8 (9,326) | 1.47 (932) |
| 65−74 | 18.0 (4,952) | 2.17 (597) |
| 75−84 | 29.0 (4,042) | 4.53 (631) |
| ≥85 | 45.4 (2,856) | 11.4 (718) |
| Total | 9.14 (29,382) | 1.01 (3,254) |
Each year in the United States, pneumococcal disease accounts for a substantial number of cases of invasive and non-invasive disease including meningitis, bacteremia, pneumonia, and acute otitis media (AOM).[3–8] A recent analysis estimated that pneumococcal disease was responsible for 4 million illness episodes, 445,000 hospitalizations and 22,000 deaths annually.[9] Pneumococcal disease is preceded by asymptomatic colonization of the nasopharynx which tends to be especially common in children.[10] AOM is the most common clinical manifestation of pneumococcal infection among children and the most common outpatient diagnosis resulting in antibiotic prescriptions in that group.[11]
Background
Trends in invasive pneumococcal disease
Following the introduction of PCV7 in 2000, dramatic declines in IPD were reported among children <5 years of age. Before introduction of PCV7, rates of PC7-type IPD among children in this age were around 80 cases per 100,000 population. After the introduction of PCV7, rates of disease due to these 7 serotypes dropped dramatically to less than 1 case per 100,000 by 2007 (Figure 1).[12]
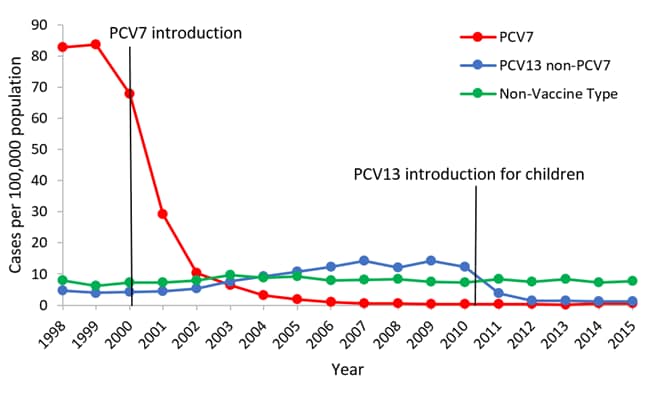
Figure 1. Rates of invasive pneumococcal disease among children <5 years of age, 1998–2015[3]
The use of PCV7 in children <5 years of age also reduced the burden of IPD among older children and adults through reduced transmission of vaccine serotype pneumococci (herd protection). In 1998–99, rates of PCV7-type IPD among adults 65 years of age or older were around 40 cases per 100,000 population. After the introduction of PCV7, rates of disease due to these 7 serotypes had declined 45% by 2007 (Figure 2).[12–16]
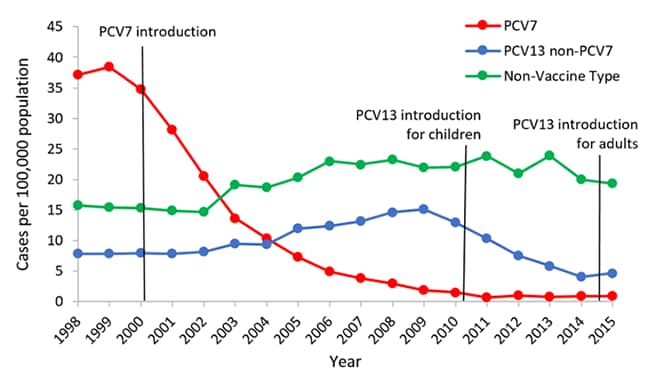
Figure 2. Rates of invasive pneumococcal disease among U.S. adults >65 years of age, 1998–2015[3]
At the same time, increases in disease caused by serotypes not included in PCV7 (i.e., replacement disease) were observed among children and adult populations, although these increases were small in magnitude compared with the overall reduction in disease.[12,17] After the introduction of PCV13 in 2010, cases of invasive disease due to the six additional serotypes covered by the vaccine saw a decrease similar to what was observed post-PCV7.[16,18] In 2007–08 (pre-PCV13), rates of PCV13-type IPD among children <5 years of age were around 14 cases per 100,000. In 2014-15 (post-PCV13), rates of PCV13-type disease had decreased by 87% (Figure 1).[3] After introduction of PCV13 in children, older adults also saw reductions in IPD through herd protection, In 2007–08, rates of PCV13-type IPD among adults 65 years of age and older were around 17 cases per 100,000. In 2014–15, rates of PCV13-type disease had decreased by 70% (Figure 2). As of 2014-15, no significant increases in non-PCV13-type IPD have been observed in any age group since the introduction of PCV13.[3] In late 2014, PCV13 was approved for routine use among adults 65 years of age or older. It will be important to continue to monitor trends in IPD as vaccination use increases among this age group.
Antimicrobial resistance trends
Before 1990, S. pneumoniae was almost uniformly susceptible to penicillin, allowing most physicians to treat persons with severe infections with penicillin alone. However, during the 1990’s, resistance to penicillin and to multiple classes of antimicrobial agents spread rapidly in the United States with an increasing trend of invasive pneumococci resistant to 3 or more drug classes.[19–22]
Following the introduction of PCV7 into the routine childhood immunization program in 2000, the incidence of antibiotic-resistant invasive disease declined substantially among both young children and older persons due to reductions in resistant infections caused by vaccine serotypes.[15,23-27] Between 1998–99 and 2008, penicillin-nonsusceptible IPD rates declined 64% for children <5 years of age and 45% for adults ≥65 years of age.[27] An increase in penicillin-nonsusceptible disease caused by serotypes not included in PCV7 was also identified during the same time period, although the magnitude of this effect remains small.[23] The prevalence of resistance varied by geographic area both before and after PCV7 introduction, with higher prevalence noted in the southeastern United States.[19,23] During 2007–08, serotypes unique to PCV13 (i.e., serotypes contained in PCV13 but not PCV7) caused 78–97% of penicillin-nonsusceptible IPD, depending on age.[27] The introduction of PCV13 in 2010 has led to further reductions in antibiotic-nonsusceptible IPD rates. From 2009–2013 rates of antibiotic-nonsusceptible IPD caused by serotypes included in PCV13, but not in PCV7, decreased by 97% among children <5 years old, and 64% among adults >65 years old.[28]
In 2008, the Clinical and Laboratory Standards Institute (CLSI) established new, higher minimum inhibitory concentration (MIC) breakpoints for defining pneumococcal susceptibility to parenterally administered penicillin when treating pneumococcal disease other than meningitis.[29] Regardless of whether the old or new parenteral penicillin breakpoints are used, penicillin-nonsusceptible IPD caused by PCV13 serotypes has decreased significantly for all age groups.[27,28]
The emergence of drug resistant S. pneumoniae (DRSP) has made treatment of pneumococcal disease more difficult. Because of a lack of rapid, sensitive, and specific diagnostic tests, therapy for pneumonia and milder illnesses such as otitis media remains empiric. In addition, groups of experts have provided national guidance for treating infections commonly caused by pneumococcus, such as otitis media and pneumonia, because of the increasing prevalence of DRSP.[30–33] Few communities exist in which resistance remains uncommon, and even in these communities, resistant infections can occur. For these reasons, clinicians and public health officials should follow national guidelines rather than attempt to create local treatment recommendations based on local resistance data. Due to the limitations of current diagnostic testing, clinicians often prescribe empiric antibacterial therapy that is not indicated or is unnecessarily broad. Inappropriate antimicrobial use contributes to the development of DRSP. Principles have been developed to encourage appropriate use of antimicrobial agents for adults and children with upper respiratory infections.[6,34–37]
Importance of Surveillance
Surveillance for invasive pneumococcal disease has four main goals:
- characterization of national and local trends,
- detection of geographic and temporal changes in the prevalence of DRSP,
- monitoring impact of vaccines on disease, and
- informing future vaccine development.
With the recent introduction of PCV13, surveillance for IPD disease among children <5 years of age is particularly important for identifying populations that may not be receiving vaccination and for monitoring the incidence of disease caused by non-vaccine serotypes (i.e., replacement disease). Surveillance for IPD disease in persons ≥5 years of age is useful to monitor the impact of PPSV vaccination, the indirect effects of PCV13, and replacement disease. Following the 2014 recommendations for PCV13 use among adults ≥65 years of age, monitoring disease trends in this age group is important to assess whether PCV13 use among adults led to further reductions in disease burden, in addition to the benefits observed through PCV13 use among children (herd effects).[16]
Serotyping of pneumococcal isolates can improve understanding of vaccine effects. However, serotyping is expensive and requires specialized reagents and extensive technical training; therefore, serotyping capacity is not widely available. The use of polymerase chain reaction (PCR) to identify pneumococcal capsular genes specific for individual capsular serotypes may be feasible for some state public health and academic research centers.[38,39] CDC’s Antibiotic Resistance Laboratory Network (ARLN) and Vaccine Preventable Disease (VPD) programs can provide serotyping assistance for state health departments. For additional information on serotyping requests, contact ARLN@cdc.gov.
Pneumococcal surveillance enables recognition of new or rare resistance patterns. Surveillance information can be used on the national level for research and policy development and at the state or local level to raise awareness of DRSP among clinicians and the general public. Surveillance data also may be useful for tracking the impact of interventions aimed at reducing unnecessary use of antimicrobial agents.
Disease Reduction Goals
Healthy People 2020 includes targets for reducing IPD among children and adults.[40] Target reduction goals for children <5 years and adults ≥65 years of age are 12 and 31 IPD cases per 100,000, respectively. In addition, Healthy People 2020 includes target goals to reduce antibiotic-resistant pneumococcal infection to 12 IPD cases per 100,000 among children <5 years of age and to 31 IPD cases per 100,000 among adults ≥65 years of age.[40] The two Healthy People 2020 targets for children and the IPD target for older adults were met in 2011, and the target for antibiotic-resistant IPD in older adults was met in 2013.[28]
Continuous surveillance is important to evaluate the impact of PCV13 on the incidence of invasive pneumococcal disease, antibiotic-resistant pneumococcal infections, and to monitor disease caused by pneumococcal serotypes not included in PCV13 (i.e., replacement disease).
Disease reduction goals also focus on minimizing complications of DRSP infections through prevention and control measures. Geographic differences in antibiotic prescribing practices have been described.[41] In sites where antibiotic prescribing is high, the proportion of IPD nonsusceptible to antibiotics is also high, suggesting that local prescribing practices may contribute to local resistance patterns.
In 1995, the CDC launched a national campaign to reduce antimicrobial resistance through promotion of appropriate antibiotic use a program that was later named Get Smart: Know When Antibiotics Work. The program continues to work with a wide variety of academic and government stakeholders, including state and local health departments, academic institutions, large healthcare systems, and healthcare professional organizations to address appropriate antibiotic use in their communities.
Case Definition
Case definitions for DRSP and IPD originally approved by the Council of State and Territorial Epidemiologists (CSTE) in 1994 and 2000, respectively.[42,43] Invasive DRSP for all ages was nationally notifiable, as was all IPD in children <5 years of age. The 2 different case definitions had separate reporting codes. To avoid the potential for duplicate notification of individual cases, the CSTE case definitions were modified in 2006 to clarify that a case should be reported only once, under a single code.[44] In 2009, the CSTE case definition was modified so that all IPD was nationally notifiable, regardless of drug resistance or the case patient’s age. Prior to 2017, case definitions for “confirmed” and “suspected” cases of IPD were specifically defined, and both were notifiable under CSTE definition.[45] Beginning in 2017, a new “probable” case classification was added to capture cases diagnosed using culture-independent diagnostic tests (CIDTs), and replaced the “suspected” case definition. The following definitions are in use for national notification of IPD in the United States.
Confirmed: Isolation of S. pneumoniae from a normally sterile site (e.g., blood, cerebrospinal fluid, or, less commonly, joint, pleural or pericardial fluid). (Event code 11723)
Probable: Identification of S. pneumoniae from a normally sterile body site by a CIDT without isolation of the bacteria.[46]
Confirmed and suspected cases of IPD should be reported to public health authorities within 1 week of diagnosis. CSTE also recommends certain clinical and epidemiological information be collected, including date of illness onset, clinical syndrome (e.g., pneumonia, meningitis), underlying medical conditions, type of diagnostic test used, and pneumococcal vaccination history. DRSP is no longer collected in national surveillance as a separate event from IPD.[45]
Laboratory Testing
Refer to Chapter 22, “Laboratory Support for Surveillance of Vaccine-Preventable Diseases” for detailed information on laboratory testing for invasive pneumococcal disease and for specific information on specimen collection and shipment.
Specimen collection
Specimen collection and shipping are important steps in obtaining laboratory diagnosis or disease confirmation. Guidelines have been published for specimen collection and handling for viral and microbiologic agents. Information is also available on using CDC laboratories as support for reference and disease surveillance; resources include:
- a central website for requesting lab testing,
- the CDC Infectious Diseases Laboratories Test Directory.
- the form [2 pages, 2.80 MB] required for submitting specimens to CDC (See Appendix 23, Form # CDC 50.34)
- information on general requirements for shipment of etiologic agents (Appendix 24 [4 pages]).
Similarly to CDC, state laboratories provide online test directories containing lists of orderable tests for that institution, along with appropriate specimen types, collection methods, specimen volume, and points of contact.
Reporting and Case Notification
Case reporting within a jurisdiction
Each state and territory (jurisdiction) has regulations and laws governing the reporting of diseases and conditions of public health importance.[47] These regulations and laws list the diseases that are to be reported, and describe those persons or institutions responsible for reporting, such as healthcare providers, hospitals, laboratories, schools, daycare and childcare facilities, and other institutions. Detailed information on reportable conditions in each jurisdiction is available through CSTE.[48]
Most jurisdictions currently require IPD to be reported to local or state/jurisdiction health authorities, regardless of the age of the patient or presence of drug resistance. Additional jurisdictions require reporting in limited populations, such as children <5 years of age. In jurisdictions with reporting requirements, confirmed and probable cases of IPD should be reported to state/jurisdiction or local health departments by healthcare providers, which may include clinicians, laboratories, hospitals, and pharmacies.[48,49] Healthcare providers should identify cases through microbiology laboratories, death certificates, hospital discharge or outpatient records, and electronic medical records. The following data are recommended for case investigation and reporting: patient’s date of birth or age, the anatomic site of specimen collection, and type of infection. Other epidemiological information that is useful includes the patient’s demographic information (e.g., sex, race and ethnicity), specimen collection date, whether the patient was hospitalized, clinical syndrome, antibiotic susceptibility, details of pneumococcal vaccination history, underlying medical conditions, daycare attendance, and outcome. Additional information may be collected at the direction of the state or local health department. The S. pneumoniae Surveillance Worksheet is included as Appendix 13 to serve as a guide for data collection during investigation of reported cases.
Case notification to CDC
Notifications for confirmed cases of IPD should be sent to CDC using event code 11723 in the National Notifiable Diseases Surveillance System (NNDSS).[45,49,50] The S. pneumoniae Surveillance Worksheet is included as Appendix 13, to serve as a guide for data collection to be included in case investigations and case notification to CDC. Case notification should not be delayed because of incomplete information or lack of confirmation. The jurisdiction in which the patient resides at the time of diagnosis should submit the case notification to CDC.
Vaccination
For specific information about pneumococcal vaccination, refer to The Pink Book, which provides general recommendations, including vaccine use and scheduling, immunization strategies for providers, vaccine content, adverse events and reactions, vaccine storage and handling, and contraindications and precautions.
Enhancing Surveillance
Several surveillance activities may improve the detection and reporting of pneumococcal disease and the quality of the reports.
Enhancing reporting of antibiotic susceptibility results
Concern over rising resistance to antibiotics has prompted many state or local health departments to increase their focus on reporting susceptibility results. CDC has worked with jurisdiction health departments to evaluate different surveillance methods to determine which methods would enhance the reliability of surveillance data, given certain goals and resource limitations.[51] Use of aggregated antibiogram data collected from all hospital laboratories within a specific area has been shown to give a relatively accurate description of the proportion of isolates that are resistant to penicillin and a limited number of other drugs,. [52] Such data, however, typically cannot be analyzed by age group or other factors of interest. Sentinel systems, which may collect individual reports with more details from a limited number of laboratories, can give an accurate view of resistance if designed well.[53]
Encouraging provider reporting
Most jurisdictions’ infectious disease surveillance systems depend upon the receipt of case reports from healthcare providers and laboratories. These data are often incomplete and may not be representative of certain populations; completeness of reporting has been estimated to vary from 6% to 90% for many of the common notifiable diseases.[47] It is important for healthcare providers to understand which events should be reported, and how critical accurate reporting is for control of communicable diseases. Increasing provider awareness of local rates of IPD and local reporting requirements may enhance surveillance.
Improving detection of DRSP in laboratories
Universal adoption of optimal testing methods and testing for resistance to recommended antibiotics would improve our ability to detect and monitor resistant pathogens.[45]
Streamlining reporting using electronic methods
Although some surveillance systems still rely on paper and pencil for data collection, use of data from sources such as electronic medical records, electronic case reporting, and laboratory information management systems can significantly improve reporting speed, enhance data quality, and reduce workload.[54–60]
Case Investigation
As with most respiratory pathogens, rapid, sensitive, and specific diagnostic tests are not available, although an assay to detect pneumococcal antigen in urine can be used to diagnose pneumococcal pneumonia or to rapidly detect pneumococcal meningitis. Early in the course of illness, diagnosis of S. pneumoniae infection is most often presumptive and the choice of antimicrobial therapy is nearly always empiric. However, once S. pneumoniae is isolated from a normally sterile body site, antimicrobial susceptibility testing may be necessary for patient management. Case investigations are not usually warranted, except in outbreaks or as determined by the state health department. CDC is available during outbreaks to assist with epidemiologic and laboratory investigations.
References
- Nuorti JP, Whitney CG. Prevention of pneumococcal disease among infants and children—use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2010;59(RR-11):1–18.
- Thigpen MC, Whitney CG, Messonnier NE, Zell ER, Lynfield R, Hadler JL, et al. Bacterial meningitis in the United States, 1998-2007. N Engl J Med 2011;364(21):2016–25. doi: 10.1056/NEJMoa1005384
- CDC. Active Bacterial Core Surveillance, Unpublished data. 2016.
- Robinson KA, Baughman W, Rothrock G, et al. Epidemiology of invasive Streptococcus pneumoniae infections in the United States, 1995–1998: Opportunities for prevention in the conjugate vaccine era. JAMA 2001;285(13):1729–35. doi: 10.1001/jama.285.13.1729
- CDC. Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 1997;46(RR-8):1–24.
- Dowell SF, Marcy SM, Phillips WR, Gerber MA, Schwartz B. Otitis media—principles of judicious use of antimicrobial agents. Pediatrics 1998;101:165–71.
- Block SL, Hedrick J, Harrison CJ, et al. Community-wide vaccination with the heptavalent pneumococcal conjugate significantly alters the microbiology of acute otitis media. Pediatr Infect Dis J 2004; 23(9):829–33. doi: 10.1097/01.inf.0000136868.91756.80
- Casey JR, Pichichero ME. Changes in frequency and pathogens causing acute otitis media in 1995-2003. Pediatr Infect Dis J 2004; 23(9):824–8. doi: 10.1097/01.inf.0000136871.51792.19
- Huang SS, Johnson KM, Ray GT, et al. Healthcare utilization and cost of pneumococcal disease in the United States. Vaccine 2011; 29(18):3398–412. doi: 10.1016/j.vaccine.2011.02.088
- Bogaert D, De Groot R, Hermans PW. Streptococcus pneumoniae colonization: the key to pneumococcal disease. Lancet Infect Dis 2004;4(3):144–54. doi: 10.1016/S1473-3099(04)00938-7
- CDC. Preventing pneumococcal disease among infants and young children: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2000;49(No. RR-9):1–35.
- Pilishvili T, Lexau C, Farley MM, et al. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis 2010;201(1):32–41. doi: 10.1086/648593
- Lexau CA, Lynfield R, Danila R, et al. Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine. JAMA. 2005; 294(16):2043–51. doi: 10.1001/jama.294.16.2043
- Moore MR, Pilishvili T, Bennett N, et al. Trends in invasive pneumococcal disease among adults, 1998–2004: implications for conjugate vaccine development. Presented at: International Conference on Emerging Infectious Diseases; 2006; Mar 19–22, Atlanta, GA.
- Whitney CG, Farley MM, Hadler J, et al. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med 2003; 348(18):1737–46. doi: 10.1056/NEJMoa022823
- Moore MR, Link-Gelles R, Schaffner W, et al. Effect of use of 13-valent pneumococcal conjugate vaccine in children on invasive pneumococcal disease in children and adults in the USA: analysis of multisite, population-based surveillance. Lancet Infect Dis 2015;15(3):301–9. doi: 10.1016/s473-3099(14)71081-3
- CDC. Direct and indirect effects of routine vaccination of children with 7-valent pneumococcal conjugate vaccine on incidence of invasive pneumococcal disease—United States, 1998–2003. MMWR Morb Mortal Wkly Rep 2005;54(36):893–7.
- Kim L, Gierke R, Lewis M, et al. Indirect effects of 13-valent pneumococcal conjugate vaccine on invasive pneumococcal pneumonia in adults. Presented at: IDWeek 2013; 2013 Oct 4, San Francisco, CA.
- Whitney CG, Farley MM, Hadler J, et al. Increasing prevalence of multidrug-resistant Streptococcus pneumoniae in the United States. N Engl J Med 2000;343(26):1917–24. doi: 10.1056/NEJM200012283432603
- Pletz MW, McGee L, Jorgensen J, et al. Levofloxacin-resistant invasive Streptococcus pneumoniae in the United States: evidence for clonal spread and the impact of conjugate pneumococcal vaccine. Antimicrob Agents Chemother 2004;48(9):3491–7. doi: 10.1128/AAC.48.9.3491-3497.2004
- Butler JC, Hofmann J, Cetron MS, Elliott JA, Facklam RR, Breiman RF. The continued emergence of drug-resistant Streptococcus pneumoniae in the United States: an update from the Centers for Disease Control and Prevention’s Pneumococcal Sentinel Surveillance System. J Infect Dis 1996;174(5):986–93. doi: 10.1093/infdis/174.5.986
- Breiman RF, Butler JC, Tenover FC, Elliott JA, Facklam RR. Emergence of drug-resistant pneumococcal infections in the United States. JAMA 1994;271(23):1831–5. doi: 10.1001/jama.1994.03510470035031
- Kyaw MH, Lynfield R, Schaffner W, Craig AS, Hadler J, Reingold A, et al. Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae. N Engl J Med 2006;354(14):1455–63. doi: 10.1056/NEJMoa051642
- Kaplan SL, Mason EO, Jr., Wald ER, et al. Decrease of invasive pneumococcal infections in children among 8 children’s hospitals in the United States after the introduction of the 7-valent pneumococcal conjugate vaccine. Pediatrics 2004;113(3 Pt 1):443– 9. doi: 10.1542/peds.113.3.443
- Stephens DS, Zughaier SM, Whitney CG, et al. Incidence of macrolide resistance in Streptococcus pneumoniae after introduction of the pneumococcal conjugate vaccine: population-based assessment. Lancet 2005;365(9462):855–63. doi: 10.1016/S0140-6736(05)71043-6
- Talbot TR, Poehling KA, Hartert TV, et al. Reduction in high rates of antibiotic-nonsusceptible invasive pneumococcal disease in Tennessee after introduction of the pneumococcal conjugate vaccine. Clin Infect Dis 2004;39(5):641–8. doi: 10.1111/bij.12587
- Hampton LM, Farley MM, Schaffner W, et al. Prevention of antibiotic-nonsusceptible Streptococcus pneumoniae with conjugate vaccines. J Infect Dis 2011;205(3):401–11. doi: 10.1093/infdis/jir755
- Tomczyk S, Lynfield R, Schaffner W, et al. Prevention of antibiotic-nonsusceptible invasive pneumococcal disease with the 13-valent pneumococcal conjugate vaccine. Clin Infect Dis 2016; 62(9):1119-25.
- Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; 18th informational Supplement. CLSI document M100-S18. Wayne, PA: Clinical and Laboratory Standards Institute; 2009.
- Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis 2011;53(7):e25–76. doi: 10.1093/cid/cir531
- American Academy of Family Physicians; American Academy of Otolaryngology-Head and Neck Surgery; American Academy of Pediatrics Subcommittee on Otitis Media With Effusion. Otitis media with effusion. Pediatrics 2004;113(5):1412–29. doi: 10.1542/peds.113.5.1412.
- American Academy of Family Physicians; American Academy of Otolaryngology-Head and Neck Surgery; American Academy of Pediatrics: Subcommittee on Management of Acute Otitis Media. Diagnosis and management of acute otitis media. Pediatrics 2004;113(5):1451–65. doi: 10.1542/peds.113.5.1451.
- Mandell LA, Bartlett JG, Dowell SF, et al. Update of practice guidelines for the management of community-acquired pneumonia in immunocompetent adults. Clin Infect Dis 2003;37(11):1405–33. doi: 10.1086/380488
- Dowell S, Marcy S, Phillips W, Gerber M, Schwartz B. Principles of judicious use of antimicrobial agents for pediatric upper respiratory tract infections. Pediatrics 1998;101(1):163–5.
- Gonzales R, Bartlett JG, Besser RE, et al. Principles of appropriate antibiotic use for treatment of uncomplicated acute bronchitis: background. Ann Intern Med 2001;134(6):521–9. doi: 10.7326/0003-4819-134-6-200103200-00021
- Gonzales R, Bartlett JG, Besser RE, et al. Principles of appropriate antibiotic use for treatment of acute respiratory tract infections in adults: background, specific aims, and methods. Ann Intern Med 2001;134(6):479–86. doi: 10.7326/0003-4819-134-6-200103200-00015
- Gonzales R, Bartlett JG, Besser RE, et al. Principles of appropriate antibiotic use for treatment of nonspecific upper respiratory tract infections in adults: background, specific aims, and methods. Ann Intern Med 2001;134(6):490–4. doi: 10.7326/0003-4819-134-6-200103200-00013
- Pai R, Gertz RE, Beall B. Sequential multiplex PCR approach for determining capsular serotypes of Streptococcus pneumoniae isolates. J Clin Microbiol 2006;44(1):124–31. doi: 10.1128/JCM.44.1.124-131.2006
- Pai R, Limor J, Beall B. Use of pyrosequencing to differentiate Streptococcus pneumoniae serotypes 6A and 6B. J Clin Microbiol 2005; 43(9):4820–2. doi: 10.1128/JCM.43.9.4820-4822.2005
- United States Department of Health and Human Services, Office of Disease Prevention and Health Promotion. Healthy People 2020—Improving the Health of Americans. Washington, DC: United States Department of Health and Human Services [cited 2017 August 16].
- Hicks LA, Chien YW, Taylor TH, Jr., Haber M, Klugman KP. Outpatient Antibiotic Prescribing and Nonsusceptible Streptococcus pneumoniae in the United States, 1996—2003. Clin Infect Dis 2011;53(7):631–9. doi: 10.1093/cid/cir443
- CSTE. National surveillance for drug-resistant Streptococcus pneumoniae (DRSP) invasive disease. CSTE position statement 1994-NSC-10. Atlanta, GA: CSTE; 1994.
- CSTE. Surveillance for invasive pneumococcal disease in children less than five years of age [3 pages]. CSTE position statement 00-ID-6. Atlanta, GA: CSTE; 2000.
- CSTE. Enhancing local, state and territorial-based surveillance for invasive pneumococcal disease in children less than five years of age [2 pages]. CSTE position statement 06-ID-14. Atlanta, GA: CSTE; 2006.
- CSTE. Enhancing state-based surveillance for invasive pneumococcal disease. CSTE position statement 09-ID-06. Atlanta, GA: CSTE; 2009.
- CDC. Invasive pneumococcal disease (IPD) (Streptococcus pneumoniae) 2017 case definition. Atlanta, GA [cited 2017 August 16].
- Roush S, Birkhead G, Koo D, Cobb A, Fleming D. Mandatory reporting of diseases and conditions by health care professionals and laboratories. JAMA 1999;282(2):164–70. doi: 10.1001/jama.282.2.164
- CSTE. State reportable conditions websites. Atlanta, GA [cited 2017 August 16].
- Jajosky R, Rey A, Park M, Aranas A, Macdonald S, Ferland L. Findings from the Council of State and Territorial Epidemiologists’ 2008 assessment of state reportable and nationally notifiable conditions in the United States and considerations for the future. J Public Health Manag Pract 2011:17(3);255–64. doi: 10.1097/PHH.0b013e318200f8da
- CSTE. Revision of the standardized case definition for invasive pneumococcal (Streptococcus pneumoniae) disease or IPD [7 pages]. CSTE position statement 16-ID-08. Atlanta, GA: CSTE; 2016.
- Noggle B, Iwamoto M, Chiller T, et al. Tracking resistant organisms: Workshop for improving state-based surveillance programs [conference summary]. Emerg Infect Dis 2006;12(3). doi: 10.3201/eid1203.051335
- Van Beneden CA, Lexau C, Baughman W, et al. Aggregated antibiograms and monitoring of drug-resistant Streptococcus pneumoniae. Emerg Infect Dis 2003;9(9):1089–95. doi: 10.3201/eid0909.020620
- Schrag SJ, Zell ER, Schuchat A, Whitney CG. Sentinel surveillance: a reliable way to track antibiotic resistance in communities? Emerg Infect Dis 2002;8(5):496–502. doi: 10.3201/eid0805.010268
- CDC. Progress in improving state and local disease surveillance—United States, 2000–2005. MMWR Morb Mortal Wkly Rep 2005;54(33:8225.
- CSTE. Improving public health practice by enhancing the public health community’s capability for electronic information exchange using HL7 CDA [5 pages]. CSTE position statement 13-SI-03. Atlanta, GA: CSTE; 2013.
- CSTE. Common data structure for national notifiable diseases. CSTE position statement 15-EB-01 [6 pages]. Atlanta, GA: CSTE; 2015.
- Smith PF, Hadler JL, Stanbury M, Rolfs RT, Hopkins RS, Group CSS. “Blueprint version 2.0”: updating public health surveillance for the 21st century. J Public Health Manag Pract 2013;19(3):231–9. doi: 10.1097/PHH.0b013e318262906e
- CSTE. Review of and recommendations for the National Notifiable Disease Surveillance System: a state and local health department perspective [49 pages]. Atlanta, GA: CSTE; 2013.
- CSTE. 2004–2010 National assessments of electronic laboratory reporting in health departments: findings and recommendations [asessment brief] [4 pages]. Atlanta, GA: CSTE; 2012.
- Mac Kenzie WR, Davidson AJ, Wiesenthal A, et al. The promise of electronic case reporting. Public Health Rep 2016;131(6):742–6. doi: 10.1177/0033354916670871