Chapter 16: Tetanus
Authors: Amy Blain, MPH; Tejpratap S. P. Tiwari, MD
Disease Description
Tetanus is an acute, potentially fatal disease that is characterized by generalized increased rigidity and convulsive spasms of skeletal muscles. Tetanus is caused by the spore-forming bacterium Clostridium tetani. Spores of C. tetani (the dormant form of the organism) are found in soil contaminated with animal and human excreta. The spores enter the body through breaks in the skin and germinate under anaerobic conditions. Puncture wounds and wounds with a significant amount of tissue injury are more likely to promote germination. The organisms produce a potent toxin, tetanospasmin, which binds to gangliosides at the neuromuscular junction and proceeds along the neuron to the ventral horns of the spinal cord or motor horns of the cranial nerves in 2–14 days. The toxin can also be absorbed into the blood stream and lymphatics. Once the toxin reaches the nervous system, it causes painful and often violent muscular contractions. The muscle stiffness usually initially involves the jaw (lockjaw) and neck, and later becomes generalized. Tetanus is a noncommunicable disease—it is not transmitted from one person to another.
Background
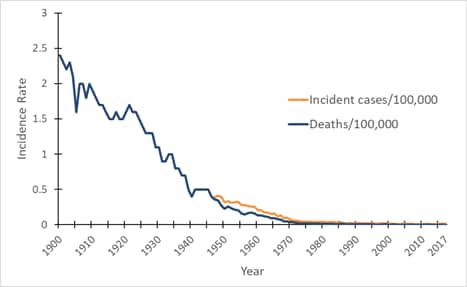
Figure 1. Mortality and incidence rates of tetanus reported in the United States, 1900–2017. view larger
*Incidence rate is calculated as cases per 100,000 population.
In the United States, reported mortality due to tetanus has declined at a constant rate since the early 1900s, and documented tetanus incidence has declined since the mid- to late 1940s, when national reporting of tetanus cases began (Figure 1). Several factors have contributed to the decline in tetanus morbidity and mortality, including the widespread use of tetanus toxoid–containing vaccines since the late 1940s. Other factors include improved wound care and postexposure use of tetanus immune globulin (TIG), either for prophylaxis in wound management or for treatment of tetanus. In addition, increased rural-to-urban migration with consequent decreased exposure to tetanus spores may also have contributed to the decline in tetanus mortality noted during the first half of the 20th century.[1]
In 2017, a total of 33 tetanus cases and 2 deaths were reported through the National Notifiable Diseases Surveillance System (NNDSS).[2] The effectiveness of tetanus toxoid-containing vaccines is very high, although not 100%.[3–5] Vaccination status was known for 72 (27%) of 264 tetanus cases reported from 2009 through 2017.[2] In only 18 (25%) was receipt of 3 or more doses of tetanus toxoid reported. The remaining patients were either unvaccinated or had received fewer than 3 doses of tetanus toxoid.
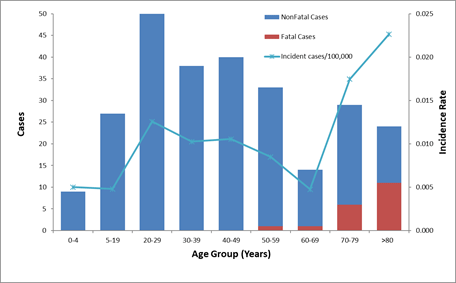
Figure 2. Number of reported cases of tetanus, survival status of patients, and average annual incidence rates by age group—United States, 2009–2017. view larger
*Incidence rate is calculated as cases per 100,000 population.
From 2009 through 2017, a total of 264 cases and 19 deaths from tetanus were reported in the United States. Sixty (23%) cases were in persons 65 years of age or older, 168 (64%) were in persons 20 through 64 years of age, and 36 (13%) were in persons younger than 20 years, including 3 cases of neonatal tetanus (Figure 2). All tetanus-related deaths occurred among patients >55 years of age.[2]
During each of these years, coverage among infants and children with at least 3 doses of DTP/DTaP/diphtheria and tetanus toxoids (DT) was 93% or higher.[6,7] Coverage with booster doses of tetanus toxoid–containing vaccine decrease with increasing age. In a 2014 survey, 62.6% of adults 19 through 49 years of age reported receiving a dose of tetanus toxoid–containing vaccine within the preceding 10 years, compared with 57.7% of adults 65 years of age or older.[8] Serologic studies of the US population correlate well with vaccination coverage and demonstrate lower immunity levels at older ages. A national population-based seroprevalence survey conducted from 1988 to 1994 found that while 20% of adolescents 12 to 19 years of age lacked protective levels of tetanus antibodies (>0.15 IU/ml), 69% of adults 70 years of age or older lacked protective levels.[9]
Diabetes, a history of immunosuppression, and intravenous drug use may be risk factors for tetanus.[10,11] From 2009 through 2017, persons with diabetes accounted for 12% of all reported tetanus cases, and 26% of all tetanus deaths. Intravenous drug users (IDUs) accounted for 8% of cases from 2009 through 2017;[2] a cluster of cases in IDUs was noted in California in the 1990s.[11]
Despite the availability of highly effective tetanus toxoid–containing vaccines, tetanus continues to have a substantial health impact in the world. In 2017, the World Health Organization (WHO) estimated that 30,848 newborns died from neonatal tetanus, a 96% reduction from the late 1980s.[12] Neonatal tetanus elimination was defined in 1993 as fewer than 1 case of neonatal tetanus for every 1,000 live births per year in each administrative district of a given country.[13] WHO and its partners (the United Nations Children’s Fund [UNICEF] and the United Nations Population Fund) are committed to eliminating maternal and neonatal tetanus. As of July 2019, 12 countries have not eliminated maternal and neonatal tetanus.[13]
Importance of Rapid Case Identification
Prompt clinical recognition of tetanus is important because hospitalization and treatment are usually required. Prompt administration of tetanus toxoid and TIG may decrease the severity of the disease. Because tetanus is an uncommon disease, consultation on clinical management may be useful.
Importance of Surveillance
Because tetanus is preventable, the possibility of failure to vaccinate should be investigated in every case. Each case should be used as a case study to determine which factors contributed to the failure, and which measures could be taken to improve the vaccine delivery system and prevent such cases in the future.
Information obtained through surveillance is used to assess national incidence and current epidemiologic trends. The information is also used to raise awareness of the importance of immunization and to characterize persons or geographic areas in which additional efforts are required to raise vaccination levels and reduce disease incidence.
Disease Reduction and Vaccine Coverage Goals
Because herd immunity does not play a role in protecting individuals against tetanus, vaccination is needed to provide individual protection. Healthy People 2020 emphasizes the importance of achieving and maintaining high levels of tetanus-containing vaccine coverage in the United States and calls for continued efforts to ensure effective tetanus vaccination coverage, with particular emphasis on children and adolescents.[14]
Case Definition
The following case definition for tetanus was approved by the Council of State and Territorial Epidemiologists (CSTE) and published in 2009.[15]
Tetanus clinical case definition
In the absence of a more likely diagnosis, an acute illness with muscle spasms or hypertonia and diagnosis of tetanus by a health care provider; or death, with tetanus listed on the death certificate as the cause of death or a significant condition contributing to death.
Case classification
Probable: A clinically compatible case, as reported by a healthcare professional.
There is no definition for confirmed tetanus.
Laboratory Testing
There is no diagnostic laboratory test for tetanus; the diagnosis is entirely clinical. C. tetani is recovered from wounds in only about 30% of cases, and the organism is sometimes isolated from patients who do not have tetanus. Serologic results obtained before TIG is administered can support susceptibility if they demonstrate very low or undetectable anti-tetanus antibody levels. However, tetanus can occur in the presence of “protective” levels of antitoxin (>0.1 IU by standard ELISA); therefore, serology cannot exclude the diagnosis of tetanus.
Reporting and Case Notification
Case reporting within a jurisdiction
Each state and territory (jurisdiction) has regulations or laws governing the reporting of diseases and conditions of public health importance.[16] These regulations and laws list the diseases to be reported, and describe those persons or groups responsible for reporting, such as healthcare providers, hospitals, laboratories, schools, daycare and childcare facilities, and other institutions. Persons reporting these conditions should contact their jurisdiction’s health department for jurisdiction-specific reporting requirements. Detailed information on reportable conditions in each jurisdiction is available through CSTE.[17] Tetanus is a reportable disease in all states and territories of the United States. The Tetanus Surveillance Worksheet is included as Appendix 18, to serve as a guide for data collection during investigation of reported cases.
Case notification to CDC
Notifications for probable and suspected cases of tetanus should be sent to the Centers for Disease Control and Prevention (CDC) using event code 10210 in the NNDSS.[18]
A provisional notification should be sent by the jurisdiction’s health department to CDC via the National Electronic Telecommunications System for Surveillance (NETSS) or National Electronic Disease Surveillance System (NEDSS), when available, within 14 days of the initial report to the jurisdiction or local health department. Supplementary information may be sent via NETSS or extended screens, or NEDSS investigation screens (see Appendix 18 [2 pages]). The Tetanus Surveillance Worksheet is included as Appendix 18, to serve as a guide for data collection during case investigations and case notifications to CDC. Reporting should not be delayed because of incomplete information. Data can be updated electronically as more information becomes available. The jurisdiction in which the patient resides at the time of diagnosis should submit the case notification to CDC.
Information to collect
The following data are epidemiologically important and should be collected in the course of case investigation. Additional information may be collected at the direction of the state health department.
- Demographic information
- Name
- Address
- State of residence
- Date of birth
- Age
- Ethnicity
- Race
- Occupation
- Reporting source
- County
- Earliest date reported
- Clinical
- Hospitalization and duration of stay
- Date of onset of symptoms
- Type of tetanus disease
- Wound location and management, including receipt of a tetanus toxoid–containing vaccine or TIG
- Complication and intensive care treatment
- Pre-existing conditions (e.g., diabetes, chronic otitis media, immunosuppression)
- Outcome (patient survived or died)
- Date of death
- Treatment
- Prophylaxis with tetanus toxoid–containing vaccine and TIG
- Date started
- Vaccine Information
- Dates of vaccination (prior tetanus toxoid–containing vaccine history)
- Number of doses of tetanus toxoid–containing vaccine received prior to infection
- Time since last dose of tetanus toxoid–containing vaccine
- Maternal vaccination (for neonatal cases)
- Epidemiologic
- Risk factors for disease (e.g., history of a wound or injury, recent injection drug use, tattooing, body piercing)
- For neonatal cases, maternal country or origin and number of years of residence in the United States
Vaccination
For specific information about the use of tetanus vaccines, refer to The Pink Book, which provides general recommendations, including vaccine use and scheduling, immunization strategies for providers, vaccine content, adverse events and reactions, vaccine storage and handling, and contraindications and precautions.
Enhancing Surveillance
A number of specific activities can improve the detection and reporting of tetanus cases and the comprehensiveness and quality of reporting. Additional activities are listed in Chapter 19, “Enhancing Surveillance.”
Promoting awareness
Efforts should be made to promote awareness among physicians and infection control practitioners to report suspected cases of tetanus promptly. The completeness of reporting of tetanus mortality to CDC has been estimated at 40%, and completeness of reporting for tetanus morbidity may be even lower.[19] Lack of direct benefits, administrative burdens, and a lack of knowledge of reporting requirements are all thought to contribute to incomplete reporting of infectious diseases by physicians and other healthcare providers.
Providing feedback
National and statewide surveillance data concerning tetanus should be regularly shared with infection control nurses, hospital epidemiologists, neurologists, and other clinicians; all should be regularly updated concerning reporting requirements. Feedback should also be provided to the persons who report cases. Representatives from jurisdiction and local health departments should attend meetings of infection control nurses and other scientific gatherings to share surveillance data and to discuss the quality and usefulness of surveillance.
Review of mortality data
Mortality data are available through the vital records systems in all states, and they may be available soon after deaths occur in states using electronic death certificates. Although the number of tetanus cases in the United States is small, each is important and warrants a full investigation. Mortality data should be reviewed each year to identify deaths that may be due to tetanus. Any previously unreported cases identified through this review should be reported. Nationally, the completeness of reporting of tetanus deaths to the vital records system is estimated at 60%.[19]
Streamlining reporting using electronic methods
Although many surveillance systems still rely on paper and pencil for data collection, use of data from sources such as electronic medical records, electronic case reporting[20–26], and clinical laboratory information systems (LIMS) can significantly improve reporting speed, enhance data quality, and reduce workload.
Case Investigation
The Tetanus Surveillance Worksheet (Appendix 18) [2 pages] may be used as a guideline for the investigation, with assistance from the jurisdiction’s health department.
References
- Roper MH, Wassilak SGF, Tiwari TSP, Kretsinger K, Orenstein WA. Tetanus toxoid. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines 6th edition. Philadelphia: Saunders; 2012:746–72.
- National Notifiable Diseases Surveillance System, 1990–2017. Division of Health Informatics and Surveillance, Center for Surveillance, Epidemiology, and Laboratory Services, Office of Public Health Scientific Services, Centers for Disease Control and Prevention, U.S. Department of Health and Human Services, Atlanta, GA 30329.
- Myers MG, Beckman CW, Vosdingh RA, Hankins WA. Primary immunization with tetanus and diphtheria toxoids: Reaction rates and immunogenicity in older children and adults. JAMA 1982;248(19):2478–80. doi:10.1001/jama.1982.03330190042028
- WHO. Tetanus vaccines: WHO position paper — February 2017. Wkly Epidemiol Rec 2017;6(92):53–76. doi: 10.1016/j.vaccine.2017.02.034
- CDC. Diphtheria, tetanus, and pertussis: recommendations for vaccine use and other preventive measures. Recommendations of the Immunization Practices Advisory Committee (ACIP). MMWR Recomm Rep 1991;40(RR-10):1–28.
- CDC. National, state, and local area vaccination coverage among children aged 19–35 months — United States, 2009. MMWR Morb Mortal Wkly Rep 2010;59(36):1171–6.
- CDC. Vaccination coverage among children 19–35 months — United States, 2017. MMWR Morb Mortal Wkly Rep 2018;67(40);1123–1128 doi: 10.15585/mmwr.mm6740a
- CDC. Surveillance of vaccination coverage among adult populations — United States, 2014. MMWR Surveill Summ 2016;65(1):1–36. doi: 10.15585/mmwr.ss6501a1
- McQuillan GM, Kruszon-Moran D, DeForest A, Chu SY, Wharton M. Serologic immunity to diphtheria and tetanus in the United States. Ann Intern Med 2002;136(9):660–6. doi: 0.7326/0003-4819-136-9-200205070-00008
- American Academy of Pediatrics. Tetanus. In: Pickering LK, editor. 2006 Red Book: Report of the Committee on Infectious Diseases, 2006. Elk Grove Village, IL: American Academy of Pediatrics; 2006:648–53.
- CDC. Tetanus among injecting-drug users — California, 1997. MMWR Morb Mortal Wkly Rep 1998;47(8):149–51.
- WHO. Immunization, vaccines and biologicals: vaccines and diseases—maternal and neonatal tetanus elimination (MNTE). Geneva: WHO [updated 2017 April 5;cited 2017 April 25]
- WHO. Expanded programme on immunization. Global Advisory Group — Part II. Wkly Epidemiol Rec 1994;69(5):29-31, 34–35.
- U.S. Department of Health and Human Services. Healthy People 2020 Washington DC: US Department of Health and Human Services [updated 2019 November 8; cited 2019 November 26].
- CSTE. Public health reporting and national notification for tetanus. CSTE position statements 09-ID-63 [8 pages]. Atlanta, GA: CSTE; 2009.
- Roush S, Birkhead G, Koo D, Cobb A, Fleming D. Mandatory reporting of diseases and conditions by health care professionals and laboratories. JAMA 1999;282(2):164–70. doi: 10.1001/jama.282.2.164
- CSTE. State reportable conditions websites. Atlanta, GA: CSTE [cited 2017 April 25].
- CDC. National Notifiable Disease Surveillance System: Tetanus 2010 case definition. Atlanta, GA: CDC [cited 2017 April 25].
- Sutter RW, Cochi SL, Brink EW, Sirotkin BI. Assessment of vital statistics and surveillance data for monitoring tetanus mortality, United States, 1979–1984. Am J Epidemiol 1990;131(1):132–42. doi: 10.1093/oxfordjournals.aje.a115466
- CDC. Progress in improving state and local disease surveillance—United States, 2000–2005. MMWR Morb Mortal Wkly Rep 2005;54(33):822–5.
- CSTE. Improving public health practice by enhancing the public health community’s capability for electronic information exchange using HL7 CDA [5 pages]. CSTE position statement 13-SI-03. Atlanta, GA: CSTE; 2013.
- CSTE. Common data structure for national notifiable diseases [6 pages]. CSTE position statement 15-EB-01. Atlanta, GA: CSTE; 2015.
- Smith PF, Hadler JL, Stanbury M, Rolfs RT, Hopkins RS, CSTE Surveillance Strategy Group. “Blueprint version 2.0”: updating public health surveillance for the 21st century. J Public Health Manag Pract 2013;19(3):231–9. doi: 10.1097/PHH.0b013e318262906e
- CSTE. Review of and recommendations for the National Notifiable Disease Surveillance System: a state and local health department perspective [49 pages]. Atlanta, GA: CSTE; 2013.
- CSTE. 2004–2010 national assessments of electronic laboratory reporting in health departments: findings and recommendations [4 pages]. [assessment brief]. Atlanta, GA: CSTE; 2012.
- MacKenzie WR, Davidson AJ, Wiesenthal A, et al. The promise of electronic case reporting. Public Health Rep 2016;131(6):742–6. doi: 10.1177/0033354916670871