What to know
Research shows that healthcare professionals are parents' most trusted source of information about the HPV vaccine. CDC encourages healthcare professionals to recommend HPV vaccination in the same way and on the same day that they recommend other vaccines for adolescents.
HPV vaccine recommendations
CDC recommends HPV vaccination for children at ages 11 or 12 years to protect against HPV infections that can cause some cancers later in life. Vaccination can be started at age 9 and is recommended through age 26 years for those who did not get adequately vaccinated when they were younger.
About HPV vaccines
9-valent HPV vaccine (Gardasil-9 [23 pages]) is a non-infectious recombinant vaccine prepared from the purified virus-like particles (VLPs) of the major capsid (L1) protein of HPV types 6, 11, 16, 18, 31, 33, 45, 52, and 58.
HPV vaccines are highly immunogenic. More than 98% of recipients develop an antibody response to HPV types included in the respective vaccines 1 month after completing a full vaccination series.
- However, there is no known serologic correlate of immunity and no known minimum titer determined to be protective.
- The high efficacy found in the clinical trials to date has precluded identification of a minimum protective antibody titer.
All HPV vaccines have been found to have high efficacy (close to 100%) for prevention of HPV vaccine type-related persistent infection, cervical intraepithelial neoplasia (CIN) 2/3, and adenocarcinoma in situ (AIS) in clinical trials in analyses limited to persons without evidence of infection with the vaccine types at the time of vaccination. The initial trials were conducted in women aged 15 or 16 through age 26 years, following a three-dose vaccination schedule. Quadrivalent vaccine was also found to have high efficacy (99%) for prevention of genital warts. Among men who have sex with men (MSM), quadrivalent vaccine had high efficacy against anal intraepithelial neoplasia grade 2 or 3 (AIN 2/3).
Immunogenicity trials conducted several years after the original vaccine licensures demonstrated that the antibody response after two doses given 6 to 12 months apart in 9- through 14-year-olds was non-inferior to the antibody response after three doses in women in the age group in which efficacy was demonstrated in the clinical trials. These studies led to approval and recommendation of a two-dose schedule in young adolescents.
For more efficacy studies, see the Pink Book Chapter on HPV.
Storage and handling
- Store refrigerated at 2 to 8°C (36 to 46°F).
- Do not freeze.
- Discard if the vaccine has been frozen.
- Protect from light.
The Vaccine Storage and Handling Toolkit is a comprehensive resource for providers on vaccine storage and handling recommendations and best practice strategies. It includes considerations for equipment, both storage units and temperature monitoring devices, strategies for maintaining the cold chain, routine storage and handling practices, inventory management and emergency procedures for protecting vaccine inventories.
Administering HPV vaccine
CDC recommends routine vaccination of preteens at ages 11 or 12 years. The vaccination series can be started at age 9 years. HPV vaccine may be given at the same time as other vaccines.
HPV vaccination is administered as:
- A two-dose series (0, 6-12 months) for most persons who initiate vaccination at ages 9 through 14 years
- A three-dose series (0, 1-2, 6 months) for persons who initiate vaccination at ages 15 through 45 years, and for immunocompromised persons.
Doses: 2
Timing: 0, 6–12 monthsA
Persons initiating vaccination at ages 9 through 14 years, except immunocompromised persons
Doses: 3
Timing: 0, 1–2, 6 monthsB
Only 9-valent HPV vaccine (Gardasil 9) has been available for use in the United States since late 2016.
Manufacturer
Merck
Year Licensed
December 2014 for males and females
HPV types protected against by vaccine
HPV types 6, 11, 16, 18, 31, 33, 45, 52, and 58
Adjuvant in vaccine
AAHS:
500 μg amorphous aluminum hydroxyphosphate sulfate
Recommended for…
- Females and males ages 11 or 12 years (can start at age 9 years)
- Persons ages 13 through 26 years who have not been adequately vaccinated when younger
Contraindicated for…
- People who had a severe allergic reaction (e.g., anaphylaxis) after a previous dose or to a vaccine component, including yeast.
The 9-valent HPV vaccine protects against HPV types 16 and 18, which cause about 66% of cervical cancers and most other HPV-attributable cancers in the United States, and five additional cancer-causing types, which account for about 15% of cervical cancers. It also protects against HPV 6 and 11, which cause most anogenital warts.
For more information on immunization schedules, see the Child and Adolescent Immunization Schedule and the Adult Immunization Schedule.
- Shake well before use. Thorough agitation immediately before administration is necessary to maintain suspension of the vaccine.
- Do not dilute or mix with other vaccines.
- After thorough agitation, HPV vaccine is a white, cloudy liquid.
- Inspect the product visually for particulate matter and discoloration prior to administration.
- Do not use the product if particulates are present or if it appears discolored.
For full instructions on dosage preparation, see the 9-valent HPV vaccine package insert.
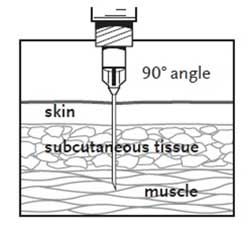
HPV vaccines should be administered intramuscularly in the deltoid region of the upper arm or in the higher anterolateral area of the thigh. The preferred site of administration is the deltoid region of the upper arm. Do not administer this product intravenously, intradermally, or subcutaneously.
- In a two-dose schedule of HPV vaccine, the minimum interval is 5 months between the first and second dose.
- In a three-dose schedule of HPV vaccine, the minimum intervals are 4 weeks between the first and second dose, 12 weeks between the second and third dose, and 5 months between the first and third dose.
