NSSP Update – January 2019
Message from the Program Manager, NSSP
The National Syndromic Surveillance Program (NSSP) had a great year because of the contributions and hard work of its Community of Practice members. The Centers for Disease Control and Prevention (CDC) takes great pride in supporting this community and in building syndromic surveillance (SyS) expertise and capacity across the country. We are particularly pleased to support the use of SyS in the ongoing fight against the opioid epidemic and natural disasters, such as hurricanes, wildfires, and earthquakes, and to expand the use of SyS for acute flaccid myelitis, suicide, and other conditions. As a new year begins, I’d like to reflect on where we’ve been, where we are, and where we’re likely headed.
On many occasions since 2014, we’ve highlighted the community’s achievements. We’ve recapped the technical improvements made to BioSense Platform tools and data flow. We’ve described the partnerships that are advancing SyS practice and shown how epidemiologists and analysts are using data in novel ways to answer public health questions. The community can take pride in NSSP’s expansion, which now includes 58 sites and represents about 64% of emergency department visits nationwide and covers all or some part of 46 states. The BioSense Platform processes 3 million messages per day into the DataMart and through ESSENCE. Further, CDC has provided capacity building funds for practitioners in 38 sites (35 states and 3 local public health departments), and these sites have had many documented successes.
History tells us that we must put effort into maintaining what we’ve achieved and into innovating to meet the next set of challenges. We now have a stable platform, useful tools, and a highly collaborative community. The NSSP has demonstrated throughout all levels of public health that syndromic data are useful, available, timely, and of high quality. We are now entering a new phase in NSSP’s trajectory —while we maintain the stability and quality we’ve worked so hard to achieve, we will continue to explore promising opportunities and address the evolving needs of a community that expects actionable data.
As resources allow, NSSP will evaluate and explore new analytic methods and data sources. We are working with the newly formed NSSP CoP Technical Committee to determine where CDC should spend precious BioSense Platform development and enhancement resources. We will improve the platform’s applications and data flow, examine machine-learning techniques that classify records into syndromes, evaluate the usefulness of adding lab data to the platform, and consider how to expand U.S. Department of Defense data sharing with states. In 2019, we will engage a Council of State and Territorial Epidemiologists (CSTE) policy group to help us work through tough questions about data access and sharing during emergency responses—What access is appropriate? What reports are needed by leadership at all levels of government and across response agencies? We will also look for better ways of filtering “noisy” statistical alerts to focus on those that are meaningful to public health, and we will work closely with the community to develop interactive data quality dashboards. We want to help our community use syndromic data in novel ways and integrate it with other surveillance methods and data to obtain the best snapshot of the public’s health—in near real-time.
Balancing these efforts will be challenging, particularly in the face of unknown future events. Who knows what the next public health emergency will bring? What seems clear, however, is that the syndromic surveillance community is strong and is committed to overcoming challenges to produce a flexible, responsive resource in the NSSP BioSense Platform, from which all public health practitioners can benefit.
I am grateful for the opportunity to represent the NSSP and join you in these pursuits.
Happy New Year! I’m certainly looking forward to it!
Sincerely,
Michael A. Coletta, MPH
National Syndromic Surveillance Program Manager
COMMUNITY OF PRACTICE UPDATES

Trending Topics
It’s flu season, and many public health departments use this time to engage with partners to increase public health awareness through reports and educational materials. If your health department generates a regular “winter hazards,” “winter surveillance,” or influenza report, please consider sharing it on the NSSP Community of Practice (CoP) Community forums. Examples of reports and targeted messages are useful resources for others looking to develop or improve their educational campaigns.
Workgroup and Committee Updates
- Overdose Surveillance Committee (ODSC): ODSC members are excited to announce that they will conduct a workshop at the 2019 International Society for Disease Surveillance (ISDS) Annual Conference in San Diego, California, in which all kinds of topics affecting the syndromic surveillance community will be explored. Please add a post to this forum thread to suggest a topic or ask a specific question you would like answered during the workshop.
- Message Guide Workgroup (MGWG): The MGWG is looking for community members, including vendors, to assist with the final recommendations being made to the proposed HL7 2.5.1 Implementation Guide for Syndromic Surveillance. Workgroup members are interested in hearing your experience and thoughts during the weekly calls (Tuesdays at 2:00 PM ET). To participate, please join the group on healthsurveillance.org or email Dave Trepanier at dtrepanier@syndromic.org to be added to the listserv and calendar series.
- Syndrome Definition Committee (SDC): If you are looking for assistance with development or validation of syndromes in ESSENCE and other syndromic surveillance systems, join the SDC conversations on Group Forums.
NSSP Community of Practice Call
Please join the monthly NSSP CoP Call. This call is powered by community members who want to share guidance, resources, and technical assistance. The NSSP CoP Call includes an open forum for discussion and questions.
The next call will be held January 15, 2019, 3:00–4:30 PM ET. We will discuss NSSP CoP activities taking place at the 2019 ISDS Annual Conference in San Diego, California. Click here to register for the entire call series.
To access slides and recordings from previous calls, visit the NSSP Community of Practice Group Page.
Implementation Guide for Syndromic Surveillance
| HL7 2.5.1 Implementation Guide Milestones | |
|---|---|
| Time Frame | Activity |
| 2015 | Completed Version 2.0 Final RELEASE* |
| 2016 | Released Erratum and Clarification Documents for Version 2.0 |
| 2017 Summer | Released Version 2.2 Working Draft for Community Comment and Consensus |
| 2017 Winter | Released Version 2.3 for Review and Community Comment |
| 2018 March | Released Version .09 |
| 2018 Spring | Submitted DRAFT HL7 Guide for Balloting: Implementation Guide for Syndromic Surveillance Release 1.0 Standard for Trial Use (STU) HL7 Version 2.5.1** |
| 2018 Fall |
|
|
|
| 2019 January |
|
| 2019 February | Submit Request to Publish Implementation Guide as a “Standard for Trial Use” |
| 2019 Spring (March-May) | 90-day Publication of Implementation Guide to HL7 Members |
| 2019 June | Release to Public: HL7 2.5.1 Implementation Guide for Syndromic Surveillance for Trial Use Version 1 |
*Version 2.0 is currently being used; subsequent versions are working drafts only.
** Added April 2, 2018.
*** Resolution of comments from Spring 2018 Ballot Process is on schedule for completion in November 2018.
CDC FUNDING RECIPIENTS AND PARTNERSHIP UPDATES
Florida’s Use of Syndromic Surveillance Identifies Unreported Cases of Zika Virus Disease
In 2016, scientists worked to understand the full extent of Zika neurological and birth defect consequences. CDC, state public health departments, and the mainstream press educated the public on what was known about Zika virus disease—its spread, link to birth defects, prevention, and geographic areas where the risk was greatest. Public health officials took what steps they could to prepare for Zika’s spread, and the Florida Health Department turned to syndromic surveillance to identify symptomatic individuals early.
With large numbers of tourists coming to Florida each year, including from many South American and Central American countries with Zika virus disease outbreaks, Florida instituted multiple measures to minimize the introduction of this disease into the state. Learn how the Florida Department of Health used syndromic surveillance to identify 17 Zika virus disease cases in 2016 and 2017 that would not have been reported to public health through traditional reporting mechanisms.
1 CDC. Zika Virus: About Zika [online]. 2017. [Cited 2018 Oct 17]. Available from URL: https://www.cdc.gov/zika/about/index.html
2 CDC. Zika Virus. Overview: Why Zika is Risky for Some People [online]. 2017. [Cited 2018 Oct 17]. Available from URL: https://www.cdc.gov/zika/about/overview.html
Louisiana Takes Action Against Drug Abuse by Sharing Syndromic Data
Like other states, Louisiana grapples with widespread drug abuse. As early as 2013, the Louisiana Office of Public Health, Infectious Disease Epidemiology section (IDEpi), began receiving requests for drug abuse data from the governor’s office and community-based organizations that needed a deeper understanding of overdose trends and populations at greatest risk. The IDEpi team, which conducts the state’s syndromic surveillance, recognized that to engage communities and organizations in the fight against drug abuse, syndromic data should be widely shared.
As Louisiana analysts and epidemiologists began sharing data, their efforts evolved into developing dashboards and other data repositories, their partners got more engaged, and they realized some unexpected benefits (for example, syndromic data helped in securing grants and other funding). This success story provides a high-level view of the pivotal role that syndromic data played in Louisiana’s surveillance of and response to drug abuse.
West Virginia Uses Syndromic Surveillance to Monitor Mass Gatherings
Surveillance capabilities are enhanced over time by trying new approaches and examining the results. In July 2017, the West Virginia Department of Health and Human Resources conducted syndromic surveillance at a 10-day event that attracted more than 30,000 youths, volunteers, and spectators.
A comprehensive plan was developed to conduct surveillance, categorize syndromes, provide treatment, and provide daily situational awareness reports. ESSENCE was used to run reports for event organizers, Department of Homeland Security, National Guard, and the governor’s office.
This was the first time for conducting syndromic surveillance at this particular event, and its duration provided good insight into the state’s health response capacity. Had a serious threat to public health occurred, syndromic surveillance would have identified syndrome patterns to allow for quick public health response and intervention. Ultimately, the surveillance results informed decisions to use syndromic surveillance to monitor upcoming global (2019) and national (2021) events.
ESSENCE QUERIES
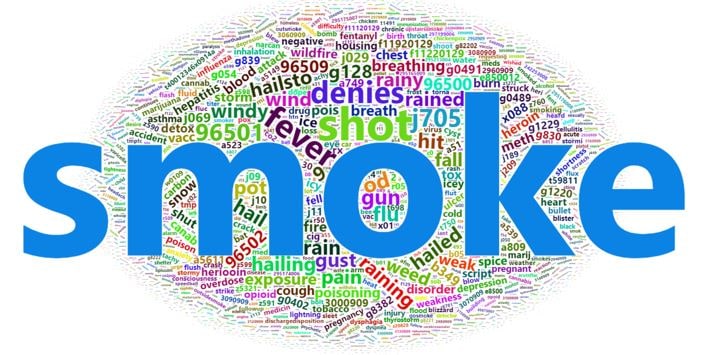
Query Terms from November 2018
We know from interacting with NSSP participants that they are doing lots of intriguing surveillance work. One way in which we can quantify and share what this activity looks like is to query ESSENCE usage logs for information about how the system is running, what queries are being performed, and how long queries take to complete (processes currently limited to NSSP super administrators). An example of the free-text and ICD code queries being run in ESSENCE can be summarized in the preceding word cloud. It was generated using the Wordcloud2 package in R. This word cloud shows the search terms that epidemiologists and data analysts used during November 2018 in their syndromic surveillance queries. The relative size of the terms suggest the frequency of use in a query.
Do you enjoy seeing national-level data depicted in a word cloud? Let us know!
CURRENT MONTH AND UPCOMING EVENTS
| January 2 | Data Validation Conference Call, 3:00–4:00 PM ET. | |
| January 15 | NSSP CoP Call; 3:00–4:30 PM ET; Topic: 17th Annual International Society for Disease Surveillance Conference; register here | |
| January 22 | Scheduled vendor patches in staging environment: 6:00–10:00 AM ET | |
| January 24 | Scheduled vendor patches in production environment: 6:00–10:00 AM ET | |
| January 31 | Deploy update to Access & Management Center | |
| January 29— February 1, 2019 |
17th Annual International Society for Disease Surveillance Conference: Harnessing Data Science to Improve Population Health and Public Health Surveillance; San Diego, California |
LAST MONTH’S TECHNICAL ASSISTANCE
| December 4 | Deployed updates to BioSense Platform (SAS Studio, SAS URL, Access & Management Center enhancements to support SAS, minor bug fixes) | |
| December 18 | Applied vendor patches in staging environment | |
| December 20 | Applied vendor patches in production environment |
DATA QUALITY CORNER
Data—the foundation for making sound public health decisions—must be managed from collection through analysis and reporting. NSSP can work with sites to assess and improve data quality. Each month, NSSP provides site-specific reports on three essential and integrated measures of data quality: completeness, timeliness, and validity. Reports can be accessed in each site’s secure shared folder and are available toward the end of the month. The Data Quality Corner can help you use these reports to bolster and maintain the integrity of your site’s data quality.
NSSP Introduces BioSense Platform Enhancements Document
The NSSP team—with considerable guidance from the NSSP CoP Technical Committee—recently developed and published the BioSense Platform Enhancements document. The Enhancements Document is designed to increase transparency by providing a historic record of enhancements being made to the AMC, data flow, and ESSENCE. Platform users will be able to view each enhancement’s review status, priority, description, and implementation stage. This living document will be updated monthly and added to the NSSP Resource Center.
We thank the NSSP CoP Technical Committee for prioritizing and clarifying enhancements that will benefit the syndromic surveillance community.
NSSP Adds Quarterly Metrics to Site Folders
Beginning this month, NSSP will add a quarterly summary of data quality metrics to each site administrator’s Reports/Production folder. This summary will include high-level visualization of the site’s data quality (processing metrics, completeness of key fields) and identify concerns that might warrant further investigation. The schedule was sent to site administers in December. Please contact your site inspector if you have questions or need help troubleshooting data quality concerns.
NSSP Updates Data Dictionary and Companion “Journeys” Guide
NSSP updated and posted the NSSP Data Dictionary (Version 33). The NSSP Data Dictionary describes each data element stored in NSSP data tables. The January updates follow:

Removal of Data Flow Enhancements Tab (enhancements are now part of the new BioSense Platform Enhancements Document)
- Processing updates related to the BioSense Platform Archive Processed Table and ESSENCE (see Table of Contents Tab, Version Notes)
The companion guidance document, Data Dictionary: Data Elements Used in NSSP Data Processing Journey, has also been updated to reflect these changes. To see these and other useful materials, visit the NSSP Resource Center.
SPOTLIGHT ON SYNDROMIC SURVEILLANCE PRACTICE

More than ever, syndromic data are being used to assess public health risks associated with mass gatherings—from detecting and investigating environmental hazards to enhancing response capacity. This article challenges public health practitioners to examine whether the infrastructure is in place to support syndromic surveillance and to ensure the surveillance mechanism being used yields timely and actionable data that justify cost.
Enhancing Surveillance for Mass Gatherings: The Role of Syndromic Surveillance1
Mass gatherings are diverse events (e.g., religious, political, sporting, cultural) affected by factors such as varying size, duration, crowd type, etc. Regardless of whether the event is high profile (Olympics), regional, or local (marathon, music festival), a public health infrastructure should be able to enhance its surveillance capacity to detect, investigate, and respond to a potential health hazard during a mass event. This is important to ensure public health and to protect the credibility and economic effects associated with the event. But is the cost of enhanced surveillance worthwhile? Will the enhanced system yield data that can be acted on quickly? This article doesn’t present syndromic surveillance as an “end all” solution. Instead, the article recommends that the limitations, operational costs, and potential benefits of conducting syndromic surveillance be carefully considered. The effectiveness of “enhanced” surveillance—frequently associated with timeliness of data and speed of transfer, data accuracy, and system flexibility—relies on a strong infrastructure. The authors state that some enhancements might, by design, benefit a single event, but that most enhancements have lasting benefit. So, how does one determine whether to use syndromic surveillance? To determine its usefulness during a mass gathering, this spotlight article proposes that four requirements be met:
- Plan for a Sustainable Investment—Integrate syndromic data into practice before the mass gathering. Syndromic surveillance should be well understood and routinely practiced. For maximum effectiveness, the surveillance system should be tested, and there should be baseline data and local context.
- Enhance Traditional Surveillance, then Integrate New Data Sources—Use syndromic surveillance in tandem with traditional surveillance sensors. For instance, syndromic surveillance can validate data being collected by traditional systems and verify the completeness and timeliness of those reports. Syndromic surveillance is but one more input that can be used to gather and make sense of data and put it in context.
- Develop Actionable Questions—Conduct a risk assessment before and during the event to identify potential public health outcomes and whether syndromic surveillance can be part of the solution. Know the strengths of syndromic data. Syndromic surveillance has higher sensitivity to some events than to others. For example, syndromic surveillance can identify heat-related illness and some injuries much more accurately than it can identify cases of infectious disease. Consequently, as new questions are asked during a mass gathering event, the system’s flexibility enables epidemiologists to add new case definitions if needed and analyze results in near real-time.
- Disseminate Accurate Interpretation—Epidemiologists and data analysts must know their data. They will be called on to put data in context for those unfamiliar with trends and sensor sensitivities, which can include other practitioners, public health officials, and the lay public. Mass gatherings have unique limitations that affect interpretation. For instance, a short event (<3 days) does not allow much time to trigger a problem, investigate it, and intervene.
The article concludes by stating syndromic data is one of many ways to enhance surveillance during a mass gathering. By meeting the four requirements described here, the likelihood of using syndromic surveillance effectively during a mass gathering will increase.
1 Fleischauer AT, Gaines J. Enhancing Surveillance for Mass Gatherings: The Role of Syndromic Surveillance. Public Health Reports [Internet]. 2017 July/August [cited 2018 Dec 26];132(1 Suppl):95S–98S. Available from: https://journals.sagepub.com/doi/full/10.1177/0033354917706343
Editor’s note. Preparedness and situational awareness involve advance planning, proven infrastructure, and data quality. For these reasons, NSSP revamped the data flow and continually monitors and works with sites to ensure data quality (completeness, timeliness, validity). NSSP supports the NSSP Community of Practice to make sure the BioSense Platform design meets routine and emergency needs and the community has a forum in which it can learn about effective practices, share tested syndromes, engage with colleagues, and access resources (forums, Knowledge Repository, expert discussions, webinars, trainings, etc.).
Any investment in the BioSense Platform goes toward long-term sustainability. The NSSP team wants users of the BioSense Platform to trust the infrastructure—and baseline data—as they work to meet the complex challenges associated with surveillance during mass gatherings. Epidemiologists and analysts who might want to use NSSP–ESSENCE to share data across public health jurisdictions will want to establish working relationships with their counterparts beforehand and set up protocols for sharing data.
Program
Revised Calculations for Estimating ED VisitsIf you missed the article about revised calculations for emergency department (ED) visit coverage, check out the December issue of NSSP Update. The community and NSSP team have put considerable effort into streamlining new-site onboarding and improving data collection, management, and reporting. Consequently, NSSP now has timely, high-quality data that can be used to estimate ED visit coverage. Look for the initial calculations in the February issue of NSSP Update. These calculations will be updated and shared quarterly. |
Active Facility Audit Begins this Month
An operational review of the Master Facility Table has revealed a number of production facilities set to “Active” are not sending data to the BioSense Platform. We want help from site administrators to determine whether these facilities need support to send data or need to be deactivated. To get started, in January 2019 we’ll share a baseline report of facilities that have not submitted visit data within 90 days. Then we’ll work with site administrators to quickly identify next steps. The new policy to deactivate facilities that fail to send data for 90 or more days begins March 2019.
NSSP to Introduce “Batch Add” Feature to MFT
Later this month, we will introduce a new feature to the AMC Master Facility Table (MFT) called “Batch Add.” Site administrators will find this feature especially useful when they want to add numerous facilities to the Master Facility Table without entering each facility one by one.
AMC Release to Support Federal Data Security Requirements
The NSSP team is working on another AMC update scheduled for mid-March. This update will respond to recent federal data security requirements. BioSense Platform users who are contractors or foreign nationals (neither a citizen nor permanent U.S. resident) must be identified as such on their user profiles. Site administrators will be required to update their users’ accounts for these two status indicators.
BioSense Platform AMC passwords will also be affected. Users will be required to follow new federal guidelines such as ensuring passwords are 12 characters long, making sure passwords are not reused for 24 cycles, and setting up new passwords where 75% of the characters used are different from those in the previous password.
The NSSP team is examining whether further changes will be needed. Stay tuned for details.
NSSP Experts to Participate at ISDS Conference
The International Society for Disease Surveillance (ISDS) conference is a premier event dedicated to advance the science and practice of biosurveillance. This year’s conference will be held in San Diego, California, on January 29 through February 1, 2019. Every year, the ISDS conference draws about 400 professionals from a broad range of disciplines to learn the latest achievements, analytic methods, best practices, conceptual frameworks, and technical innovations in the rapidly evolving field of disease surveillance.
This year’s theme—Harnessing Data Science to Improve Population Health and Public Health Surveillance—will be supported through oral, lightning, roundtable, panel, and poster presentations. Visit the Swap Meet to hear the many ways we’re improving data quality, onboarding, and more. Also, check out the workshops on topics including ESSENCE and R. The daily program is posted on the ISDS website. To follow NSSP topics of interest, check out these sessions:
Wednesday, January 30
- Presentation—What Can you Really Do with 35,000 Statistical Alerts a Week Anyways?; Michael Coletta
- Presentation—Use of ESSENCE APIs to Support Flexible Analysis and Reporting; Aaron Kite-Powell
- Poster—Updates to the HL7 2.5.1 Implementation Guide for Syndromic Surveillance; Peter Hicks
- Poster—Use of N-grams and Term Relationship Graphs in the Syndrome Definition Development Process; Nimi Idaikkar
Thursday, January 31
- Lightning Talk—Using Evaluation to Inform the BioSense Platform: Results from a 2018 Survey; Sebastian Romano
- Lightning Talk—Customizing ESSENCE Queries for Select Mental Health Sub-indicators; Achintya Dey
Friday, February 1

Roundtable—Forming Collaborations through the Data Quality Committee to Address Urgent Incidents
This roundtable is being led by Krystal Collier of the Arizona Dept. of Health Services. Participants will include Kaityln Ciampaglio of the Centers for Disease Control and Prevention; Sophia Crossen of the Kansas Dept. of Health and Environment, and Courtney Fitzgerald of Cerner.
The success of any syndromic surveillance program relies on developing a standardized, easy-to-implement process for responding to urgent data incidents. Participants will learn of scenarios where data quality processes and partnerships have had a real effect on syndromic surveillance, and they’ll benefit from hearing different points of view from various stakeholders (vendors, CDC personnel, NSSP Community of Practice members). Participants can give their input and be involved in a brainstorming session that will help to develop a standard operating procedure for data quality that will be used toward future preparedness.