May 2023
May 25, 2023, 3:00-4:00 PM ET: eSHARE special session: “Reconciliation and finalization of 2022 NNDSS mpox annual data.”
June 25–29, 2023: Council of State and Territorial Epidemiologists (CSTE) 2023 Annual Conference in Salt Lake City, UT. Register for the conference.
Last Friday of each month, 1:00-2:00 PM ET: Monthly CSTE Data Standardization Work Group meetings. Join the CSTE Data Standardization Work Group.
Process for 2022 Annual Reconciliation and Finalization
On May 16, 2023, CDC and reporting jurisdictions began the process of reconciling 2022 National Notifiable Diseases Surveillance System (NNDSS) annual data.
Modernizing the reconciliation process
As part of ongoing efforts to modernize public health data, CDC has streamlined the reconciliation module in the Message Validation, Processing, and Provisioning System (MVPS) portal. The streamlined reconciliation module offers expanded functionality, augmented secure data exchange features, and organized process flows.

Jurisdictions can now conduct reconciliation completely within the MVPS portal. This change increases transparency, reduces email communication, and improves control for jurisdictions as they work through the reconciliation process.
Jurisdictions should ensure that staff involved in reconciliation have access to the MVPS portal, including State and Territorial Epidemiologists and Sexually Transmitted Disease (STD) Data Managers. Jurisdiction Data Managers can request access for their staff by emailing the CDC Electronic Data Exchange mailbox at edx@cdc.gov with the subject: “New MVPS User Access.”
Key milestones for 2022 reconciliation
CDC will work closely with your jurisdiction’s reporters to reconcile 2022 NNDSS data over the next few months. Please encourage subject matter experts in your jurisdiction to actively collaborate with CDC disease-specific programs to identify and resolve any data issues.
Your final electronic approval is due in the MVPS portal no later than August 25, 2023. You will not be able to make further modifications to data for the NNDSS tables after your final approval.
Finalizing 2022 COVID-19 data
CDC will use the same process as last year to finalize 2022 coronavirus disease 2019 (COVID-19) data:
- The jurisdiction will enter final aggregate 2022 COVID-19 counts using a web form hosted on the CDC Epi Info™ Secure Web Survey system.
- Each jurisdiction will receive a unique link to the Epi Info™ web form to submit final 2022 aggregate COVID-19 counts.
- The State or Territorial Epidemiologist will use the Epi Info™ web form to sign off and submit data to CDC.
Finalizing 2022 mpox data
Finalization of the 2022 mpox data will follow this process:
- CDC will use case data submitted through CDC’s Data Collation and Integration for Public Health Event Response (DCIPHER) for reconciliation.
- Data may be reviewed in DCIPHER, and a mechanism will be available to lock and finalize the 2022 mpox data.
- CDC will use these data for the 2022 annual NNDSS tables.
An eSHARE special session on May 25th at 3:00 PM ET will provide additional details on the process to reconcile the 2022 mpox data.
NNDSS surveillance officers are here to help!
NNDSS Surveillance Officers will help guide you through the process of reconciliation and will e-mail reconciliation packets to reporters in your jurisdiction.
If you have questions about the updates to MVPS or need help reconciling or finalizing your 2022 annual data, please contact your jurisdiction’s NNDSS Surveillance Officer listed below:
- Alan Schley (aso7@cdc.gov): AZ, DC, DE, KY, MT, NJ, NY, OH, OK, SD, UT, WI, U.S. territories
- Courtney Cook (uns6@cdc.gov): CO, FL, HI, IA, MD, MN, ND, NM, NV, RI, SC, WA, WV
- Diana Onweh (onw1@cdc.gov): AR, CT, GA, ID, IL, IN, LA, MI, MS, NH, PA, TN, TX, WY
- Keaton Hughes (qwy4@cdc.gov): AK, AL, CA, KS, MA, ME, MO, NC, NE, NYC, OR, PR, VA, VT
CDC updates case surveillance modernization FAQ webpage

Last month, the NNDSS team updated CDC’s Frequently Asked Questions (FAQs) for Case Surveillance Modernization. This page provides answers to common questions about implementing enhancements to case surveillance.
Q: Which message mapping guides (MMGs) are exceptions to the pause, and how is CDC making that determination?
A: There are three exceptions to the pause in MMG onboarding:
- Jurisdictions already in later stages of pre-onboarding or onboarding for any MMG.
- Generic version 2 (Gen v2) MMG: Jurisdictions should continue to implement Gen v2 for the 72 conditions previously approved to be sent through Gen v2.
- Tuberculosis/latent tuberculosis infection (TB/LTBI) MMGs: Jurisdictions should continue to implement the TB/LTBI MMG.
Fully supporting the TB/LTBI MMG is important to accomplish the transition from the 2009 Report of Verified Case of Tuberculosis (RVCT) to the 2020 RVCT. Jurisdictions have invested in implementing the new MMG to meet the deadline to use the 2020 RVCT for surveillance year 2023 cases. There is no legacy case notification format that includes the 2020 RVCT content, so any jurisdiction not using the TB/LTBI MMG will need to manually enter TB/LTBI MMG data starting in early 2023. To avoid this manual entry burden on jurisdictions, CDC will continue to onboard the TB/LTBI MMG for all jurisdictions.
CDC is working with jurisdictions to understand their MMG implementation status. We expect to provide specific guidance to each jurisdiction on which MMGs should be paused or will be supported for onboarding.
Q: How can jurisdictions use people who were hired for MMG implementation work while MMG development and onboarding are on pause?
A: The best approach for reassigning staff will differ by jurisdiction and the expertise of the individuals in surveillance, vocabulary, data exchange, or system design. CDC encourages you to consider the following activities for staff hired for MMG implementation:
- Support implementation of TB/LTBI MMG, GenV2 MMG, and jurisdiction-specific exceptions to the pause on MMG onboarding.
- Work with CDC and CSTE to standardize and harmonize data elements.
- Support changes to the case surveillance system that would enable the jurisdiction to submit core data elements to NNDSS during an emergency response.
- Support efforts to modernize the jurisdiction’s case surveillance system.
- Engage in jurisdiction efforts to automate case surveillance data exchange and interoperability (e.g., electronic case reporting, electronic laboratory reporting, inter-jurisdiction exchange of case reports, interoperability with an immunization information system and death registries, master person index, and data exchange through a third-party platform).
- Plan and implement changes to the surveillance system.
Q: How should jurisdictions use the content in disease-specific MMGs to guide decisions about data elements and value sets when building out disease-specific data in their surveillance systems?
A: Use the current condition-specific MMGs like a data dictionary. They present the data elements and associated value sets that CDC programs request for national surveillance. The MMGs can be used to inform decisions about the data elements in your surveillance system.
Jurisdictions and CDC programs should expect some changes to disease-specific data elements as public health works to standardize elements across the case surveillance ecosystem and to harmonize data elements across conditions. This process will take time as jurisdictions, CDC programs, and other partners collaborate on the decision making.
We invite state, tribal, local, territory, and CDC programs to engage in the standardization of case-surveillance content by joining the CSTE Data Standardization Work Group. CSTE holds meetings of the Data Standardization Work Group the last Friday of each month at 1:00 PM ET. Contact Becky Lampkins at blampkins@cste.org to learn more.
Questions or feedback?
Contact CDC using this form if you have questions for CDC or ideas for future updates to the FAQs.
Learn more about Case Surveillance Modernization.
Want to create the future of case surveillance?
CDC and the CSTE Data Standardization Work Group need your help to make case surveillance more efficient by standardizing data across the case surveillance ecosystem.
CSTE holds meetings of the Data Standardization Work Group the last Friday of each month at 1:00 PM ET.
CDC held an eSHARE webinar, titled “Kick-off for reconciliation and finalization of 2022 NNDSS, COVID-19, and mpox annual data,” on May 16, 2023. Access the slides and recording in the eSHARE archive. CDC posts these materials soon after each webinar.
CDC will hold an eSHARE special session, titled “Finalization of 2022 mpox annual data,” on May 25, 2023, 3:00-4:00 PM ET.
To join an eSHARE webinar, please see your calendar invitation or contact edx@cdc.gov for login information with the subject line “eSHARE invitation
Although CDC has paused most disease-specific MMG development and onboarding efforts as part of modernizing case surveillance, we encourage jurisdictions to implement and onboard the GenV2 and TB/LTBI MMGs.
HAI MDRO MMG
The PHIN Vocabulary Access and Distribution System (VADS) Healthcare-Associated Infections (HAI), Multidrug-Resistant Organisms (MDRO) Case Notification View Version 4 is now available for the HAI MDRO MMG version 1.0.2. The changes include:
- 2 values added to auris (now v.2).
- 2 values added to PHVS_LabTestType_CP-CRE (now v.2).
- 4 values added to PHVS_LabTestMethod_MDRO (now v.3).
- 16 values added and five deprecated for PHVS_GeneName_CP-CRE (now v.4).
- 76 values added and 2 deprecated for PHVS_Organism_CP-CRE (now v.3).
Malaria MMG
The PHIN VADS Malaria Case Notification View version 6 is now available for the Malaria MMG version 1.0.2. This view updates the value set for Travel Reason (Malaria) to version 4 by adding 10 additional values and deprecating one.
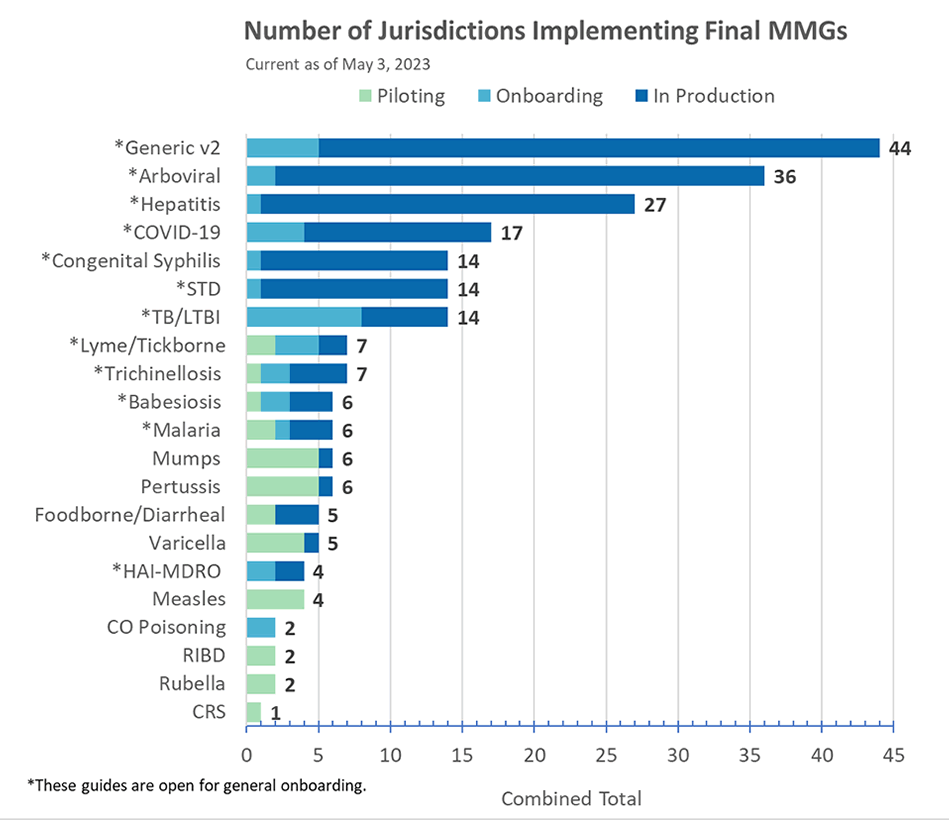
Congratulations to the following jurisdictions for being in production as of May 3!
- Maryland for Congenital Syphilis MMG
- Kentucky for Malaria MMG
- Iowa for STD MMG
- Minnesota and Pennsylvania for TB MMG
Spotlight: NNDSS at the 2023 CSTE Annual Conference
CSTE will hold its annual conference on June 25-29, 2023. The conference connects public health epidemiologists from across the country to share best practices. Mark your calendars for the following presentations to get important updates and insights about NNDSS!
All presentations and posters listed below are in the surveillance/informatics track.
Visualizing Case Surveillance and Emergency Department Data to Support the National 2022 Mpox Public Health Emergency Response, May-December 2022
- Format: Oral presentation
- Presenting: Shannon Kindilien, CDC Case Surveillance Operations Team
- Monday, June 26, 3:45−5:00 PM MT
National Notifiable Diseases Surveillance System (NNDSS) Progress and Future Directions
- Format: Oral Presentation
- Presenting: Sara Johnston, NNDSS Program Lead
- Tuesday, June 27, 3:45−5:00 PM MT
Moving Towards Standardized Data Elements for Case Surveillance
- Format: In-person roundtable
- Presenting: Danielle Tack and Amanda Jones, CDC Data Standardization and Assistance Team
- Wednesday, June 28, 12:45−1:30 PM MT