November 2022
December 15, 2022: EXTENDED Deadline to:
- sign off on final 2021 reconciliation data and
- send National Notifiable Diseases Surveillance System (NNDSS) final aggregate 2021 COVID-19 case counts.
January 17, 2023, 3:00—4:00 PM ET: January eSHARE Webinar: “What Jurisdictions Need to Know for 2023.” There will not be an eSHARE webinar in December 2022.

Case surveillance modernization updates
CDC is excited to share several updates on our efforts to enhance case surveillance based on findings from a recent discovery sprint led by a joint team from the United States Digital Service (USDS) and CDC. This effort supports the vision outlined in CDC Moving Forward and CDC’s Data Modernization Initiative (DMI).
New case surveillance modernization webpage
CDC has posted a new “Case Surveillance Modernization” section of the NNDSS website, including:
- a high-level overview of CDC’s current implementation plans for each recommendation,
- frequently asked questions for program and technical audiences, and
- a report with USDS/CDC findings from the case surveillance discovery sprint.
We invite you to bookmark these webpages and check back regularly for the latest updates. CDC will continue to update this website as new information is ready to share.
Case surveillance modernization endorsement
On November 1, 2022, CDC’s IT & Data Governance (ITDG) endorsed modernization of NNDSS case surveillance data flow changes to enable state, territorial, local, and tribal public health jurisdictions to send case data flexibly.
ITDG’s endorsement is an important accomplishment for the implementation team that will help accelerate adoption, promote awareness, and ensure compliance across the agency.
Questions and feedback
We’ll continue to share updates for this effort through our regular communication channels, such as the NNDSS newsletter Case Surveillance News and eSHARE webinars.
If you have technical or implementation-related questions and feedback, please use this jurisdiction feedback form.
General questions and feedback should be directed to dmi@cdc.gov with the subject line “Case Surveillance Enhancements.”
Thank you for your collaboration as we all undertake this critical work for public health!
CDC to stop accepting two syphilis event codes in 2023

Starting January 1, 2023, CDC will no longer accept event codes 10314 (syphilis, late latent) and 10319 (syphilis, late with clinical manifestations) for new syphilis cases.
Active event codes for syphilis case notifications are aligned with the 2018 Council of State and Territorial Epidemiologists (CSTE) case definition and should be used. They include:
- 10311 (primary),
- 10312 (secondary),
- 10313 (early non-primary, non-secondary),
- 10316 (congenital), and
- 10320 (unknown duration or late).
Beginning with cases reported for Morbidity and Mortality Weekly Report (MMWR) year 2023, jurisdictions should not use codes 10314 and 10319 to submit data to NNDSS for new syphilis cases. New 2023 cases sent to CDC with event codes 10314 and 10319 will receive an error message and will not be published in the weekly and annual NNDSS data tables on CDC WONDER and data.CDC.gov.
Jurisdictions may continue using event codes 10314 and 10319 to update cases already sent to CDC for previous years.
If you have questions about CDC retiring event codes 10314 and 10319, contact CDC’s STD program at std_surv_inquiry@cdc.gov.
Learn how CDC calculates MMWR weeks and years.
For technical support with the Message Validation, Processing, and Provisioning System, contact edx@cdc.gov.
Actions for jurisdictions: updated case definitions for Chlamydia and Gonorrhea
Chlamydia case definition
In 2021, CSTE approved position statement 21-ID-06 to update the standardized case definitions for chlamydia case reporting and national notification for use beginning in 2022. CDC has implemented the 2022 chlamydia case definition and posted it on the NNDSS website.
Beginning in MMWR week 1 of 2022, jurisdictions should ensure that chlamydia cases meeting the current CSTE case definition are transmitted to CDC with the “confirmed” case classification status.
CDC will publish only confirmed chlamydia cases meeting the updated case definition in the 2022 weekly and annual NNDSS data tables on CDC WONDER and data.CDC.gov. CDC will not publish 2022 chlamydia cases classified as probable, possible, suspect, unknown, or not a case.
Gonorrhea case definition
In 2022, CSTE approved position statement 22-ID-03 to update the standardized case definitions for gonorrhea case reporting and national notification for use beginning in 2023. CDC is working to implement the 2023 gonorrhea case definition and post it on the NNDSS website.
Beginning in MMWR week 1 of 2023, jurisdictions should send new 2023 gonorrhea cases to CDC only if they meet the criteria for “confirmed” or “probable” case.
CDC will publish only confirmed and probable gonorrhea cases meeting the updated case definition in the 2023 weekly and annual NNDSS data tables on CDC WONDER and data.CDC.gov. CDC will not publish 2023 gonorrhea cases classified as possible, suspect, unknown, or not a case.
Learn more
Contact CDC’s STD program at std_surv_inquiry@cdc.gov if you have questions about the case definitions for chlamydia or gonorrhea.
Visit CDC’s searchable library of current and historical case definitions to learn more about case definitions.
Learn how CDC calculates MMWR weeks and years and find an MMWR weeks calendar for 2021–2022.
NNDSS event codes for four new nationally notifiable conditions in 2023
Based on position statements approved by CSTE in 2022, CDC has created event codes for conditions that will become nationally notifiable or come under standardized surveillance in 2023.

Jurisdictions should plan to use the following event codes for these nationally notifiable conditions in 2023:
- 50270: Carbapenemase-Producing Organisms, clinical—CDC is seeking Office of Management and Budget (OMB) approval to receive data on this condition through NNDSS
- 50271: Carbapenemase-Producing Organisms , screening—CDC is seeking OMB approval to receive data on this condition through NNDSS.
- 50264: Candida auris, screening—As of January 1, 2023, the event name “Candida auris, screening” will replace “Candida auris, colonization/screening”; the event code 50264 will remain the same.
- 11585: Melioidosis—This is a new notifiable condition beginning January 1, 2023. NNDSS has OMB approval to receive data for this condition.
- 12036: Strongyloidiasis (current or past)—CDC is seeking OMB approval for NNDSS to receive data on this condition, which CSTE put under standardized surveillance but did not make nationally notifiable.
CDC encourages jurisdictions to begin planning to include these event codes in your surveillance information systems so that you will be ready to send these conditions through NNDSS in 2023.
If you have questions about these event codes, please contact your jurisdiction’s assigned NNDSS surveillance officer listed below.
Need help during 2021 annual reconciliation? Have questions about event codes or case definitions? NNDSS Surveillance Officers are here to help!
- Keaton Hughes (qwy4@cdc.gov): AL, AK, CA, HI, KS, ME, MA, MN, MO, NE, NC, ND, NYC, OR, PR, SC, VT, VA
- Diana Onweh (onw1@cdc.gov): AR, CO, CT, GA, ID, IA, IL, IN, LA, MD, MI, MS, NH, NV, PA, TN, TX, WA, WY
- Alan Schley (aso7@cdc.gov): AZ, DE, FL, KY, MT, NJ, NM, NY, OH, OK, RI, SD, UT, DC, WV, WI, territories
The November eSHARE, which provided information about NNDSS event code updates for 2023, emergency response case data elements, and implementation updates for CDC case surveillance enhancements, was held November 15, 2022. Visit the eSHARE archives to access the slides and recording for the November webinar.
The next eSHARE webinar, “What Jurisdictions Need to Know for 2023,” will be held January 17, 2023, 3:00—4:00 PM ET. There will not be an eSHARE webinar in December 2022.
To join an eSHARE webinar, please see your calendar invitation or contact the CDC Electronic Data Exchange mailbox at edx@cdc.gov for login information with the subject line “eSHARE invitation.”
CDC is pausing most disease-specific (supplemental) message mapping guide (MMG) development and onboarding efforts. Learn more about how CDC is modernizing case surveillance.
COVID-19 Message Mapping Guide
Version 1.2 of the COVID-19 message mapping guide has now posted to the NNDSS website, which adds the data element Lineage (INV1405).
The PHIN VADS COVID-19 Case Notification View version 13 is now available for COVID-19 MMG versions 1.0, 1.1, and 1.2. The changes include:
- 37 additional values to Risk Factor (COVID-19) (now v.2),
- 13 additional values to Treatment Type (COVID-19) to include medication treatment options, including Paxlovid (now v.2),
- 12 additional values (6 new U.S. vaccines and 6 non-U.S.) to Vaccine Type (NND) (now v.11), and
- one non-COVID additional value for Manufacturers of vaccines (MVX) (now v.14).
Additionally, version 1.2 of the “COVID Lite” test case scenarios worksheet and associated test scenarios have been posted to the NNDSS website. This updated version incorporates both Lineage (INV1405) and First Specimen Collection Date (95366-1) in addition to the GenV2 and vaccine data elements.
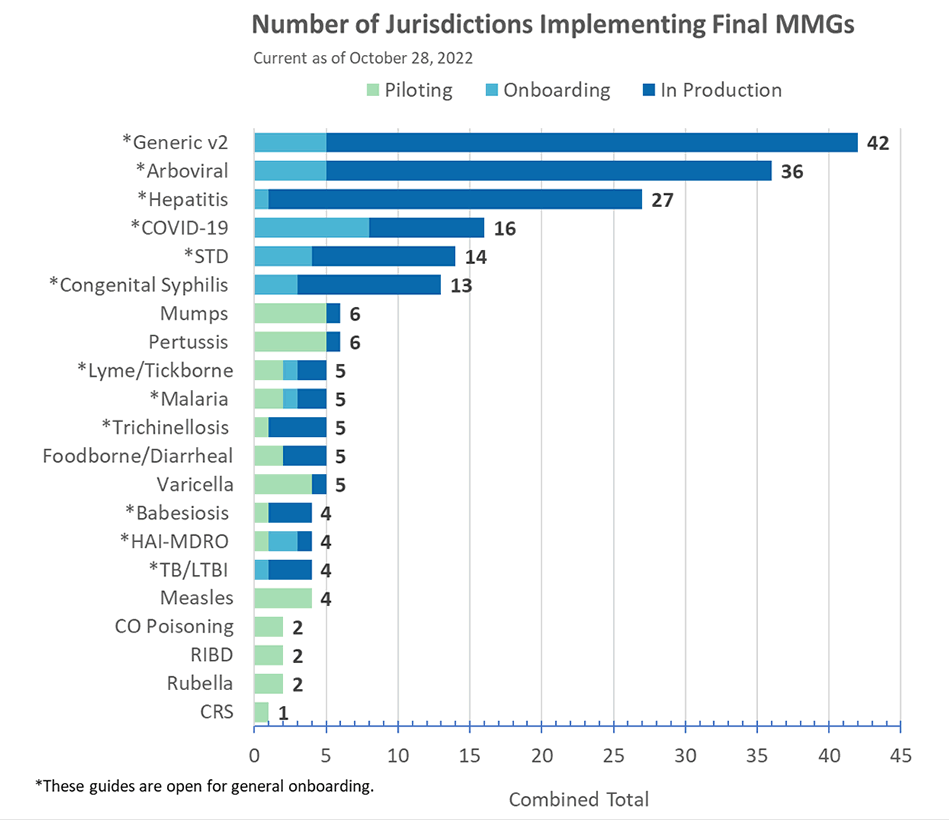
Congratulations to Michigan for being in production for COVID-19 MMG!
Reminder: Upcoming enhancements for NNDSS annual data on CDC WONDER
CDC’s Wide-ranging Online Data for Epidemiologic Research (WONDER) and the National Notifiable Diseases Surveillance System are planning to release a new interactive tool for users of NNDSS annual data on CDC WONDER.
A key data source for public health, the NNDSS annual tables provide information on cases of selected infectious national notifiable diseases and conditions sent to CDC by U.S. states and territories. These enhancements are part of CDC’s efforts to modernize public health data systems.
The new interactive tool will:
- Allow users of NNDSS annual data to create data visualizations customized to their needs.
- Offer more flexibility to focus their analyses and understand trends and patterns, such as comparing NNDSS data across years, specific populations, diseases and conditions, and geographic areas.
- Enhance user experience compared to the current, fixed format annual data tables.
- Make NNDSS data more transparent.
With the release of these new interactive features, CDC is not adding any new data elements to NNDSS data displays or the ability to stratify existing NNDSS data elements in ways not previously available.
CDC expects to release the new interactive tool in winter 2022 and will provide a more specific release date soon.
Access the presentation slides and recording for the October 18, 2022, eSHARE webinar in our eSHARE webinars archive.
Contact cwus@cdc.gov if you have questions about these upcoming features.