June 2022
June 19–23, 2022: Council of State and Territorial Epidemiologists (CSTE) Annual Conference, Louisville, Kentucky. Learn more
June 28, 2022, 3:00–4:00 PM ET: eSHARE special session: Modernizing the National Electronic Disease Surveillance System (NEDSS) Base System (NBS).
CDC is modernizing the NBS disease surveillance platform
An important component of the public health data modernization initiative is the work to modernize NBS.
NBS modernization will impact all jurisdictions. This project is not just about updating one surveillance system. NBS modernization will benefit the entire case surveillance ecosystem by building new technical components and standards for challenges faced by current surveillance systems. Solutions developed through this effort will serve as a model for improvements to other systems. New services and functionality developed during the NBS modernization will be shared so that all jurisdictions can benefit from them.

Modernizing NBS is critical to achieving data modernization goals. The end goals for NBS modernization include:
- implementing a stable, secure, easily deployable integrated disease surveillance system that ingests all actionable information from electronic laboratory reporting (ELR), electronic case reporting (eCR), and other formats such as for vital records and immunization data;
- providing jurisdictions easy access to analysis-ready data that are enriched with metadata and integrate with analytical and visualization tools;
- balancing completeness, accuracy, timeliness, and flexibility of data based on specific surveillance goals;
- offering an easy-to-learn, intuitive interface with sophisticated case, chronic disease, and outbreak management tools;
- adding more useful workflow automations;
- adding the capability to report HIV surveillance data to CDC;
- supporting integrations with third-party apps or plug-ins as part of a public health data and software ecosystem; and
- continuing to evolve to meet the changing needs of jurisdictions.
CDC plans to release small, frequent, easy-to-implement updates, and new features to incrementally modernize NBS. At the end of this project, the current NBS will be transformed into a modernized system. Benefits of this approach include:
- minimizes disruption and risk to current NBS users,
- gives NBS users immediate benefits from smaller changes over time so users will not have to wait for the development and testing of an entirely new system to benefit from the enhancements,
- provides an opportunity to add new features and services while transitioning to a fully modernized NBS, and
- allows for fast and easy rollback of system changes if there are any issues with an upgrade or new feature.
Successful modernization depends on including the diverse perspectives of all jurisdictions throughout the development process. This includes jurisdictions that haven’t implemented NBS but are considering NBS as part of a modernization plan. It is critical that this effort incorporates a wide variety of perspectives from jurisdictions, CDC programs, and key partners such as CSTE and Association of Public Health Laboratories (APHL).

CDC leadership is confident that modernizing NBS will benefit everyone involved in the case surveillance data flow. CDC is investing an unprecedented amount of support for this effort. We are excited about the future of this project and the opportunity it provides to strengthen the entire public health data infrastructure. Although there will be challenges to overcome and risks to be aware of, CDC leadership is committed to identifying the resources needed to accomplish these goals.
Join us on June 28, 2022, 3:00–4:00 PM ET, for an NNDSS eSHARE webinar that will provide information about the plans for NBS modernization. Learn more about this webinar in the eSHARE webinars section below.
Send CDC your feedback on NBS modernization at nbs@cdc.gov.
Update on 2021 annual reconciliation process
The process for reconciliation of 2021 annual NNDSS data will be similar to the reconciliation process for 2020 NNDSS data. CDC expects to begin 2021 annual reconciliation in August 2022.
CDC will not reconcile COVID-19 data. Instead, we will collect aggregate 2021 NNDSS COVID-19 data using an Epi Info™ web form similar to the one used for 2020 COVID-19 data. State and territorial epidemiologists will sign off on aggregate 2021 COVID-19 case counts within the Epi Info™ web form.
What you can do now:
- Continue cleaning your 2021 data to prepare for the start of reconciliation.
- Download case line lists using the Message Validation, Processing, and Provisioning System (MVPS)
- Send case notification updates to correct your data as appropriate.
- Finalize low-incidence case verification responses and send case notification updates to accurately reflect responses indicating “Do Not Publish.”
What to expect:
- NNDSS surveillance officers will send each jurisdiction a copy of their reconciliation/stratification tables.
- Review the line lists and the reconciliation/stratification tables and inform the CDC surveillance officer assigned to your jurisdiction to lock your data when correct.
- After CDC locks your data, your surveillance officer will send you:
- updated reconciliation/stratification tables and
- the final signature tables for the state or territorial epidemiologist to sign and return to CDC.
- Return your jurisdiction’s final signature tables before the end of reconciliation. CDC expects to finish 2021 reconciliation by early December 2022.
Surveillance officers for 2021 reconciliation:
- Keaton Hughes (qwy4@cdc.gov): AK, AL, CA, HI, KS, MA, ME, MN, MO, NC, ND, NE, NYC, OR, PR, SC, VA, VT
- Diana Onweh (onw1@cdc.gov): AR, CO, CT, GA, IA, ID, IL, IN, LA, MD, MI, MS, NH, NV, PA, TN, TX, WA, WY
- Alan Schley (aso7@cdc.gov): AZ, DC, DE, FL, KY, MT, NJ, NM, NY, OH, OK, RI, SD, UT, WV, WI, territories
CDC provides event code to help jurisdictions track Mpox cases
The public health response to the current mpox outbreak continues to evolve. Case surveillance is important for describing the disease, risk factors, and populations impacted in the U.S. and abroad.
Jurisdictions are encouraged to use event code 11801 to track mpox cases within their surveillance information systems. Use of event code 11801 will be required when data can be sent through NNDSS. However, please do not report mpox cases though routine NNDSS methods at this time.
Find the latest mpox surveillance case definition, how to send case data to CDC, and other resources on the Information for Health Departments webpage. CDC will update this webpage as the response evolves.
Learn how eCR benefits everyone involved in case reporting

Electronic case reporting is the automated, real-time exchange of case report information between electronic health records and public health agencies. It moves data quickly, securely, and seamlessly from EHRs in healthcare facilities to state and local public health agencies.
eCR benefits everyone involved in case reporting. eCR provides timely and more complete data than manual reporting and decreases the burden on both healthcare facilities and public health staff.
Other eCR benefits for public health agencies include:
- Provides data to support outbreak management and monitor disease trends.
- Efficiently monitors the spread of reportable diseases during outbreaks and public health emergencies.
- Reduces response time with automated information.
- Improves communication and collaboration with healthcare by enabling bidirectional data exchange.
- Supports submission of case-based surveillance data without identifiable information to CDC through the National Notifiable Diseases Surveillance System.
Ready to learn more?
Access the eCR Getting Started webpage.
Contact the eCR team at ecr@cdc.gov.
On June 28, 2022, 3:00–4:00 PM ET, CDC will hold a special eSHARE session about plans for modernizing NBS.
This webinar will provide an update on:
- why CDC is modernizing NBS;
- how NBS advances data modernization goals;
- how CDC plans to modernize NBS;
- how CDC will include the diverse perspectives of jurisdictions, CDC programs, and key partners such as CSTE and APHL throughout the development process;
- how NBS modernization can benefit current NBS jurisdictions, jurisdictions considering implementing NBS, and the entire case surveillance data ecosystem; and
- how long NBS modernization will take.
To join an eSHARE webinar, please see your calendar invitation or contact the CDC Electronic Data Exchange mailbox at edx@cdc.gov for login information with the subject line “eSHARE invitation.”
Jurisdictions cannot update case notification details for low-incidence conditions within the MVPS portal.
If you need to make an update, you will need to transmit a case notification to MVPS with the relevant change.
For example, if you need to update a case to “not a case” after marking it as “do not publish” in the low-incidence case verification module, you will need to transmit a case notification to update MVPS.
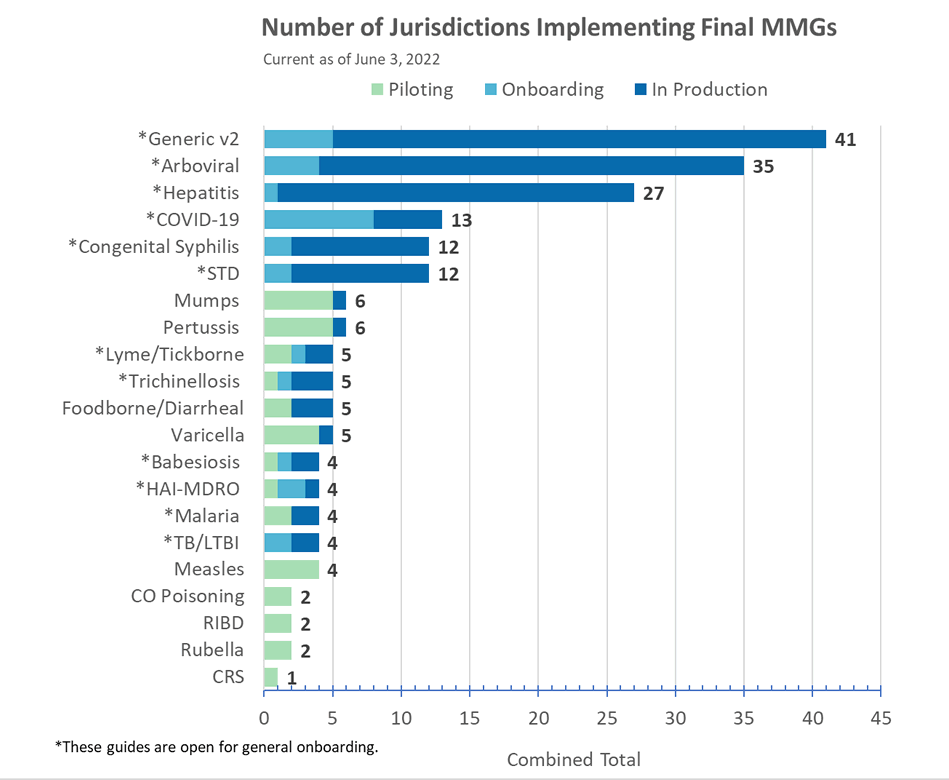
Lyme and Tickborne Rickettsial Diseases (TBRD) MMG
Version 1.0.2 of the Lyme and TBRD MMG has now posted. This update includes formatting updates being applied across all guides. Additionally, version 3 of the PHIN VADS Lyme and TBRD Case Notification View is now available. The changes include:
- spelling correction of a value in Organism (Lyme) for Organism Name (LAB278) in Lyme,
- the most current Country (ISO) value set for Travel Country (82753-5) in TBRD, and
- 18 additional tests in Lab Test Type (TBRD) for Test Type (INV290)
If you are interested in joining a cohort or onboarding an MMG, please email the CDC Electronic Data Exchange mailbox at edx@cdc.gov.
Congratulations to the following jurisdictions in production as of June 3, 2022!
- Minnesota for Arboviral MMG
- New York for FDD MMG
CDC updates process for low-incidence verification of vancomycin-resistant Staphylococcus aureus cases
CDC has updated the process for verifying cases of vancomycin-resistant Staphylococcus aureus.
These cases now require only jurisdiction review before publication in weekly NNDSS tables. Previously, these cases also required CDC program review.
CDC-verified counts will be included as a footnote to the weekly NNDSS tables.
Email us at edx@cdc.gov if you have questions about this change.
Spotlight: 2022 CSTE Annual Conference

CSTE will hold its annual conference on June 19–23, 2022. The conference connects public health epidemiologists from across the country to share best practices. Mark your calendars for the following presentations and posters to get important updates and insights about NNDSS!
All presentations and posters listed below are in the surveillance/informatics track.
Efforts to Standardize and Align Core Data Elements in Case Notification Message Mapping Guides (MMGs) with Electronic Case Reporting (eCR) and United States Core Data for Interoperability (USCDI) Format
- Format: In-person roundtable
- Presenting: Danielle Tack and Bill Morrill
- June 20, 7:30–8:15 AM ET
Case-based and Syndromic Surveillance Data Visualization in R
- Format: On-demand lightning presentation
- Presenting: Xia Michelle Lin for George Arthur
- June 20, 9:05–9:10 AM ET
NNDSS Progress and Future Directions
- Format: Breakout, virtual presentation
- Presenting: Sara Johnston
- June 20, 4:00–5:15 PM ET
Applying Power Business Intelligence (BI) Techniques to National Notifiable Diseases Surveillance Data Visualizations
- Format: Breakout, virtual presentation
- Presenting: Yusheng Zhai
- June 20, 4:00–5:15 PM ET
Identifying Training Needs and Improvements to the National Notifiable Diseases Surveillance System (NNDSS) Health Level 7 (HL7) Case Notification Process
- Format: In-person roundtable
- Presenting: Amanda Jones and Michele Hoover
- June 21, 7:30–8:15 AM ET
Advancing National Notifiable Diseases Surveillance System Data Visualizations
- Format: On-demand lightning presentation
- Presenting: Xia Michelle Lin
- June 21, 9:00–9:05 AM ET
Modernized Low-Incidence Verification of Case-Based Reporting for the National Notifiable Diseases Surveillance System
- Format: In-person poster presentation
- Presenting: Keaton Hughes
- June 21, 3:15–4:00 PM ET
New and Improved Approach for Annual Reconciliation of NNDSS Data
- Format: Virtual poster presentation
- Presenting: Keaton Hughes
- Available On-Demand