What is Radiation? The Electromagnetic Spectrum
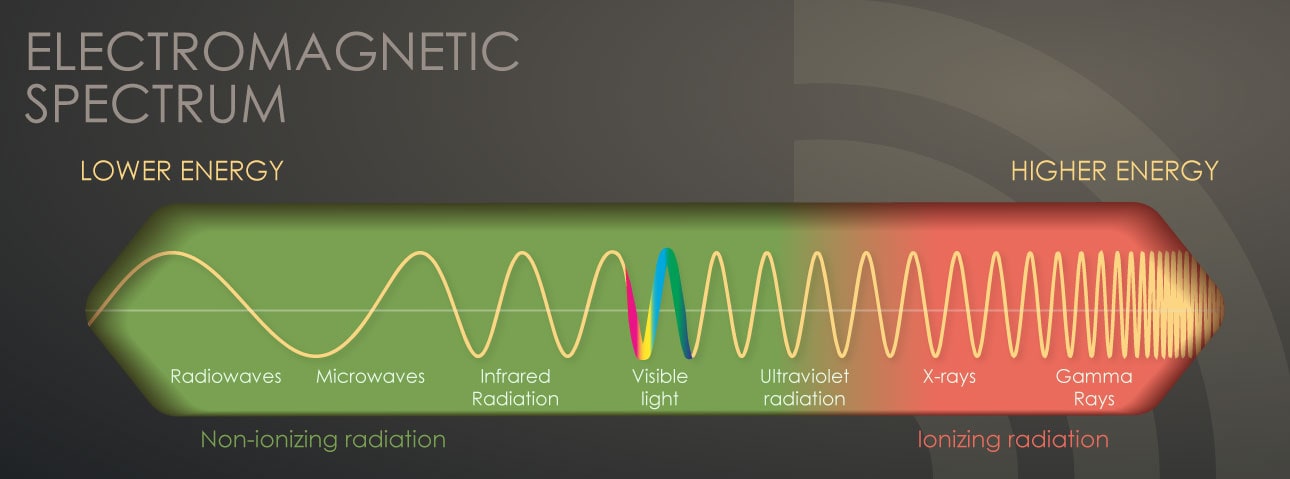
The most common form of radiation we are all familiar with is visible light. Light is energy that originates from a source and travels through space at the speed of … light! It has a particular wavelength and frequency that defines its energy.
We can detect this radiation with our eyes. The only difference between various colors of light, red, yellow, green, blue, and purple is in their wavelength or frequency, or in other words in their energy. Red light, for example, has less energy than purple light.
There is a wide range of electromagnetic radiation in nature. The visible part of the spectrum is only a tiny part of this wide range of energies.
As we move down in frequency from red light, there are other familiar forms of electromagnetic radiation:
- Infrared
- Microwaves
- Signals from our cell phones
- Radio waves
These are all forms of radiation that are invisible to our eyes and that have less energy than visible light.
As we move up in frequency from purple light, there are
These are all forms of radiation with energies much higher than visible light.
X-rays and gamma rays have enough energy that during interaction with atoms, they can remove electrons and cause the atom to become charged or ionized. That’s why we refer to these as ionizing radiation. When most people talk about radiation, they are referring to ionizing radiation.
For more information on ionizing radiation, click here
Ionization is a unique property that other forms of radiation at lower frequencies, such as those from our cell phones, do not have.
For more information on non-ionizing radiation, click here
To learn more about what happens to an ionized atom, click here
Frequency – How rapidly waves move or ‘oscillate’ up and down. The lower the frequency of the radiation, the lower its energy.
Ionizing radiation — any radiation capable of displacing electrons; from atoms, thereby producing ions. High doses of ionizing radiation may produce severe skin or tissue damage. See also alpha particle,beta particle,gamma ray,neutron,x-ray.
Ionization – the process of adding one or more electrons to, or removing one or more electrons from atoms or molecules, thereby creating ions. High temperatures, electrical discharges, or nuclear radiation can cause ionization.
Radiation — energy moving in the form of particles or waves. Familiar radiations are heat, light, radio waves, and microwaves. Ionizing radiation is a very high-energy form of electromagnetic radiation.
Wavelength – Distance covered by one complete cycle of the electromagnetic wave. In other words, the distance from one peak to another peak or from trough to trough in one wave. The longer the wavelength of the radiation, the lower its energy.