How Can I Help My Patients with HIV Start Treatment?
- Antiretroviral therapy (ART) is recommended for all people with HIV, regardless of CD4 cell count.
- Assess your patients’ readiness to begin ART.
- Help your patients adhere to ART by engaging in regular conversations at every office visit.
- Monitor your patients’ viral load to confirm initial and sustained response to ART.
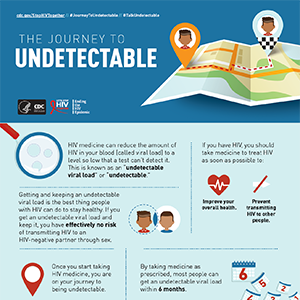
ART should be started as soon as possible after diagnosis and should be accompanied by patient education regarding the benefits and risks of ART and the importance of adhering to ART. People with HIV who are aware of their status should be prescribed ART and, by achieving and maintaining an undetectable (<200 copies/mL) viral load, can remain healthy for many years.1
ART reduces HIV-related morbidity and mortality at all stages of HIV infection and reduces HIV transmission.2,4-10 ART is known to reduce levels of multiple markers of immune activation and inflammation. Mortality associated with uncontrolled HIV replication at higher CD4 counts is believed to be due to immune activation and an inflammatory milieu that promotes progression of end-organ disease. In the large, multinational Strategic Timing of Antiretroviral Treatment (START) study,4 asymptomatic patients with HIV infection who started ART immediately after diagnosis had a >50% reduction in morbidity and mortality compared with patients who deferred treatment until they had a CD4 count ≤350 cells/mm3.
ART reduces the risk of transmitting HIV to others. Another compelling reason for treating HIV at all CD4 counts is to prevent HIV transmission. Three landmark studies have shown that treatment prevents sexual transmission of HIV.4,5,11 These trials support the fact that people with HIV who take ART as prescribed and achieve and maintain an undetectable viral load won’t transmit HIV through sex.12 This is known as treatment as prevention and is discussed in further detail in “How Does Treatment as Prevention Benefit My Patients with HIV? ”.
- When taken consistently as prescribed, ART can: 2,3
- Suppress viral load.
- Maintain high CD4 cell counts.
- Prevent the most advanced stage of HIV.
- Prolong survival.
- Reduce the risk of transmission to others.
Risk of HIV transmission with undetectable viral load by transmission category
| Transmission category | Risk for people who keep an undetectable viral load |
| Sex (oral, anal, or vaginal)13,14 | Studies have shown no risk of sexual transmission |
| Pregnancy, labor, and delivery15 | 1% or less* |
| Sharing syringes or other drug injection equipment16 | Unknown, but likely reduced risk |
| Breast/chestfeeding17 | Substantially reduces, but does not eliminate, risk** |
**Individuals who have questions about breast/chestfeeding or who desire to breast/chestfeed should receive patient-centered, evidence-based counseling on infant-feeding options. Visit CDC’s webpage on HIV and pregnancy, infants, and children for more information.
Recent findings show that assessing a patient’s ART readiness is the first step to successful ART adherence.11 Patients starting ART should be willing and able to commit to treatment and understand the benefits and risks of therapy and the importance of adherence.
When assessing a patient’s readiness to begin ART, objectivity and a nonjudgmental attitude are important. Make it clear that even if you do not share your patients’ views, you respect them. Showing that you understand and respect your patients’ views helps to strengthen your relationships with your patients and encourages patients to be open with you and to adhere to their treatment.
In the image below are some examples of questions you can use to assess your patients’ ART readiness:

If a patient isn’t ready to commit to ART treatment, provide information on other interventions that can reduce HIV transmission risk and keep them in care using the techniques detailed in “How Do I Keep Patients with HIV in Care?”.
Adherence to ART over the long term can be challenging, even for the most motivated patients. Talking to your patients with HIV makes a difference. You can positively impact ART adherence among people with HIV by engaging in regular conversations at every office visit to identify ART adherence barriers, offer adherence support services, and provide information on other interventions that can improve patient adherence and reduce the risk of HIV transmission to others.18 One way you can enhance communication is to ask your patients open-ended questions. The questions in the image below can help you better understand each patient’s views, barriers, and ability to adhere to their treatment regimen.

Once the conversation has started, you may find that your patients are encountering barriers to adherence. Below is a list of common barriers and ways you can address them through routine conversations during patient visits.
In addition to supporting ART adherence, monitoring your patients’ viral loads is an important aspect of HIV care. A patient’s plasma HIV RNA viral load should be measured regularly to confirm initial and sustained response to ART. Most patients taking ART as prescribed achieve viral suppression within 6 months.
Note that patients may experience temporary increases or “blips” in their viral load, defined as viral loads transiently detectable at low levels. These blips usually go back down by the next viral load test. Patients who are using viral suppression as their primary prevention method and experience a blip should use other prevention strategies until their viral load is undetectable again. These prevention strategies could include condoms and pre-exposure prophylaxis (PrEP) for partners without HIV.
- InitiationWith initiation of ART (before initiation and within 2 to 4 weeks after treatment initiation, followed by 4- to 8-week intervals until the levels become undetectable).
- ModificationAfter ART modification because of suboptimal response (within 2 to 4 weeks after treatment modification, followed by 4- to 8-week intervals until the levels become undetectable).
- Regimen ChangeAfter ART modification because of toxicity or need for regimen simplification (within 4 to 8 weeks after changing therapy).
- Stable RegimenIn patients on a stable, suppressive ART regimen (every 3 to 4 months, or every 6 months if virally suppressed for more than 2 years, to confirm durable viral suppression).
- Suboptimal ResponseIn patients with suboptimal response (frequency depends on clinical circumstances).
1 Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. Department of Health and Human Services. Accessed November 17, 2020. https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/adult-adolescent-arv/guidelines-adult-adolescent-arv.pdf
2 Bavinton B, Grinsztejn B, Phanuphak N, et al, for the Opposites Attract Study Group. HIV treatment prevents HIV transmission in male serodiscordant couples in Australia, Thailand and Brazil. Presented at the 9th IAS Conference on HIV Science; July 25, 2017; Paris, France. http://programme.ias2017.org/abstract/abstract/5469
3 O’Brien WA, Hartigan PM, Martin D, et al. Changes in plasma HIV-1 RNA and CD4+ lymphocyte counts and the risk of progression to AIDS. Veterans Affairs Cooperative Study Group on AIDS. N Engl J Med. 1996;334(7):426-431. https://www.nejm.org/doi/full/10.1056/NEJM199602153340703
4 Garcia F, de Lazzari E, Plana M, et al. Long-term CD4+ T-cell response to highly active antiretroviral therapy according to baseline CD4+ T-cell count. J Acquir Immune Defic Syndr. 2004;36(2):702-713. https://journals.lww.com/jaids/fulltext/2004/06010/long_term_cd4__t_cell_response_to_highly_active.7.aspx
5 INSIGHT START Study Group. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373(9):795-807. https://www.nejm.org/doi/full/10.1056/NEJMoa1506816
6 TEMPRANO ANRS Study Group, Danel C, Moh R, et al. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med. 2015;373(9):808-822. https://www.nejm.org/doi/full/10.1056/NEJMoa1507198
7 Kitahata MM, Gange SJ, Abraham AG, et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med. 2009;360(18):1815-1826. https://www.nejm.org/doi/full/10.1056/NEJMoa0807252
8 Mofenson LM, Lambert JS, Stiehm ER, et al. Risk factors for perinatal transmission of human immunodeficiency virus type 1 in women treated with zidovudine. Pediatric AIDS Clinical Trials Group Study 185 Team. N Engl J Med. 1999;341(6):385-393. https://www.nejm.org/doi/full/10.1056/NEJM199908053410601
9 Wood E, Kerr T, Marshall BD, et al. Longitudinal community plasma HIV-1 RNA concentrations and incidence of HIV-1 among injecting drug users: prospective cohort study. BMJ. 2009;338:b1649. https://www.bmj.com/content/338/bmj.b1649
10 Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493-505. https://www.nejm.org/doi/full/10.1056/NEJMoa1105243
11 Reynolds SJ, Makumbi F, Nakigozi G, et al. HIV-1 transmission among HIV-1 discordant couples before and after the introduction of antiretroviral therapy. AIDS. 2011;25(4):473-477. https://journals.lww.com/aidsonline/Fulltext/2011/02200/HIV_1_transmission_among_HIV_1_discordant_couples.9.aspx
12 Hardy, WD. The art of ART adherence: a common-sense approach to helping patients adhere to life-saving drug therapy. HIV Specialist. 2012;4(3):30-32. https://aahivm.org/wp-content/uploads/2017/03/HIVspecialist_Oct_2012_FINAL.pdf
13 Centers for Disease Control and Prevention. Evidence of HIV treatment and viral suppression in preventing the sexual transmission of HIV. Accessed August 27, 2021. https://www.cdc.gov/hiv/risk/art/evidence-of-hiv-treatment.html
14 Rodger AJ, Cambiano V, Bruun T, et al., for the PARTNER Study Group. Sexual activity without condoms and risk of HIV transmission in serodifferent couples when the HIV-positive partner is using suppressive antiretroviral therapy. JAMA. 2016;316:171-1811. https://jamanetwork.com/journals/jama/article-abstract/2533066?casa_token=C7mS84STqxYAAAAA:fpCmVNevXAccZf7JU-L9PDh3IjvENqfE17bqM1oed6CPP7BA4sxhlL3WmejJI2qaucFMCxs2Rw
15Panel on Treatment of HIV in Pregnancy and Prevention of Perinatal Transmission. Recommendations for the use of antiretroviral drugs during pregnancy and interventions to reduce perinatal HIV transmission in the United States. Accessed February 3, 2023 https://clinicalinfo.hiv.gov/en/guidelines/perinatal/whats-new
16 Wood E, Milloy MJ, Montaner JS. HIV treatment as prevention among injection drug users. Curr Opin HIV AIDS. 2012;7:151-156. https://journals.lww.com/co-hivandaids/Abstract/2012/03000/HIV_treatment_as_prevention_among_injection_drug.10.aspx
17 Townsend CL, Cortina-Borja M, Peckham CS, de Ruiter A, Lyall H, Tookey PA. Low rates of mother-to-child transmission of HIV following effective pregnancy interventions in the United Kingdom and Ireland, 2000–2006. AIDS. 2008;22(8):973-981. https://journals.lww.com/aidsonline/Fulltext/2008/05110/HIV_infected_pregnant_women_and_vertical.00008.aspx
18 Bogart LM, Mutchler MG, McDavitt B, et al. A randomized controlled trial of Rise, a community-based culturally congruent adherence intervention for black Americans living with HIV. Ann Behav Med. 2017;51(6):868-878. https://academic.oup.com/abm/article/51/6/868/4648599