JYNNEOS Vaccine Additional Considerations for Intradermal Administration
Intradermal (ID) Administration
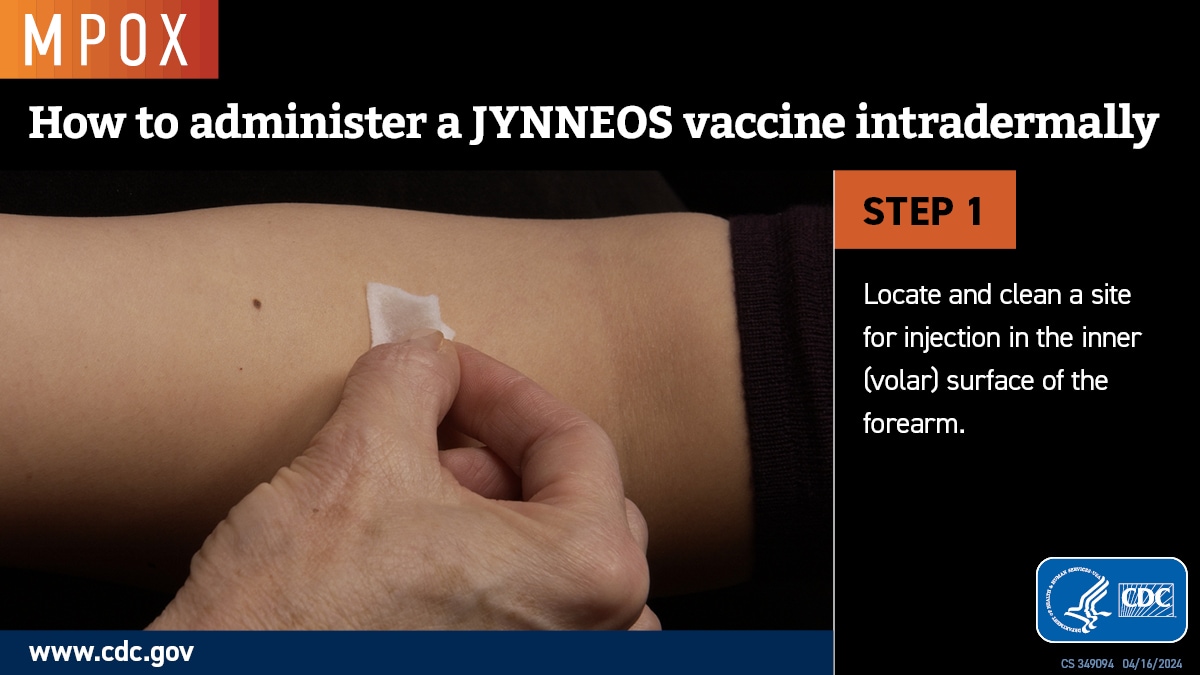
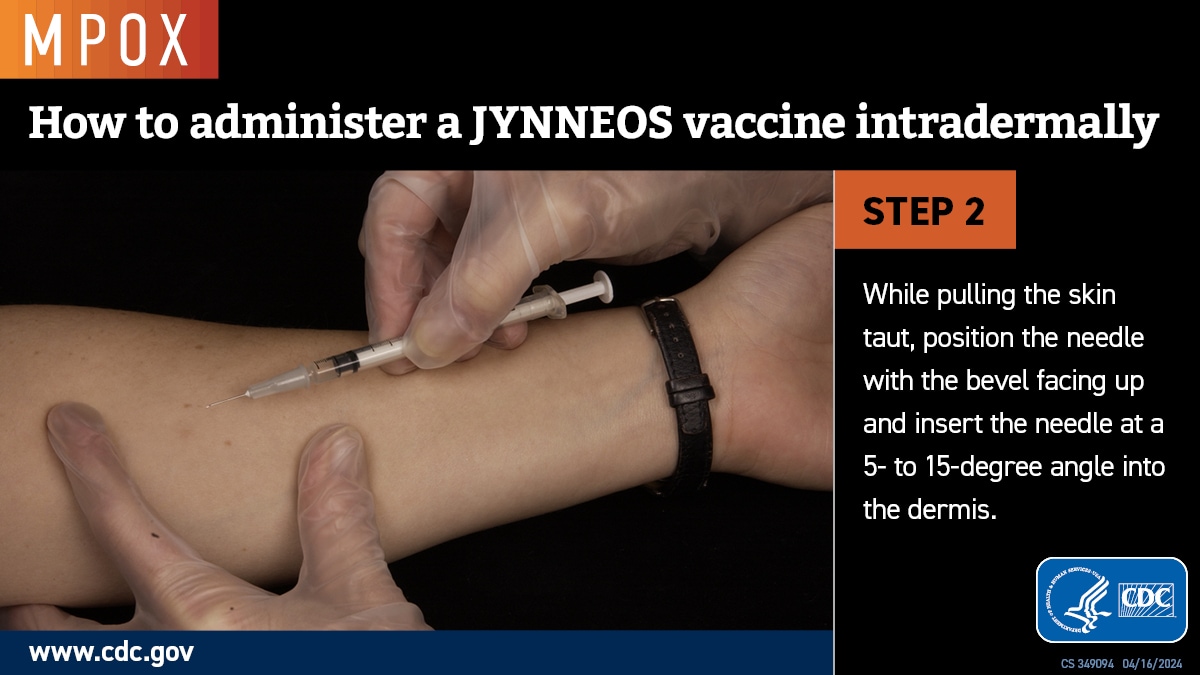
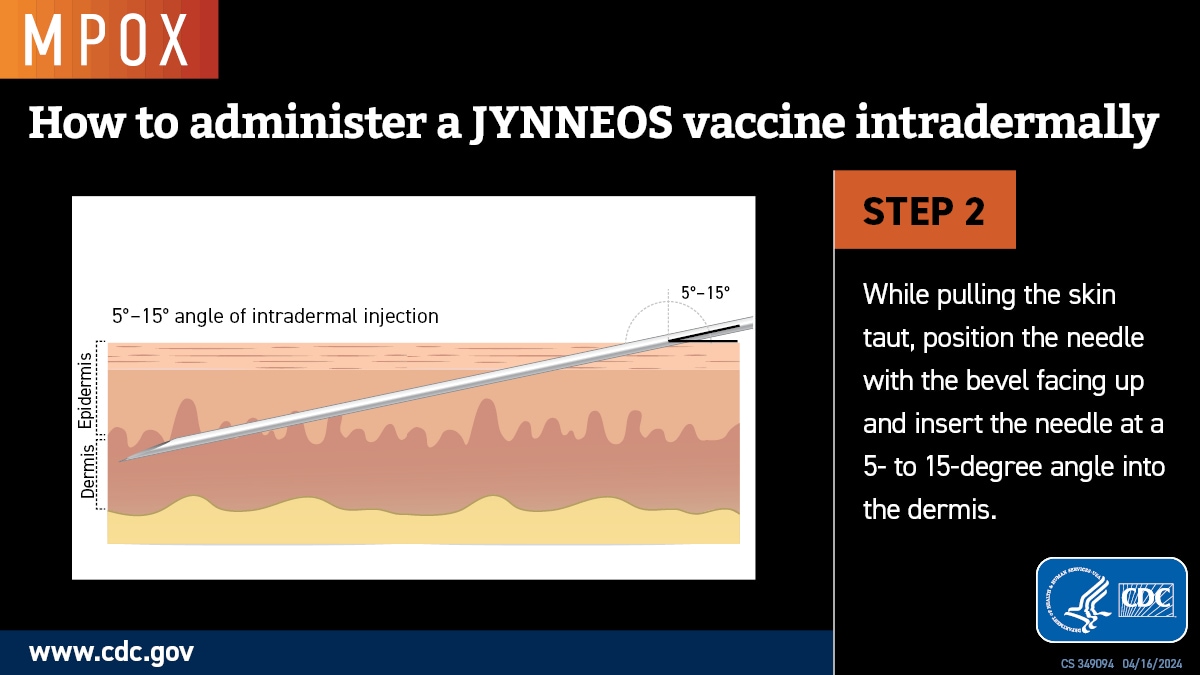
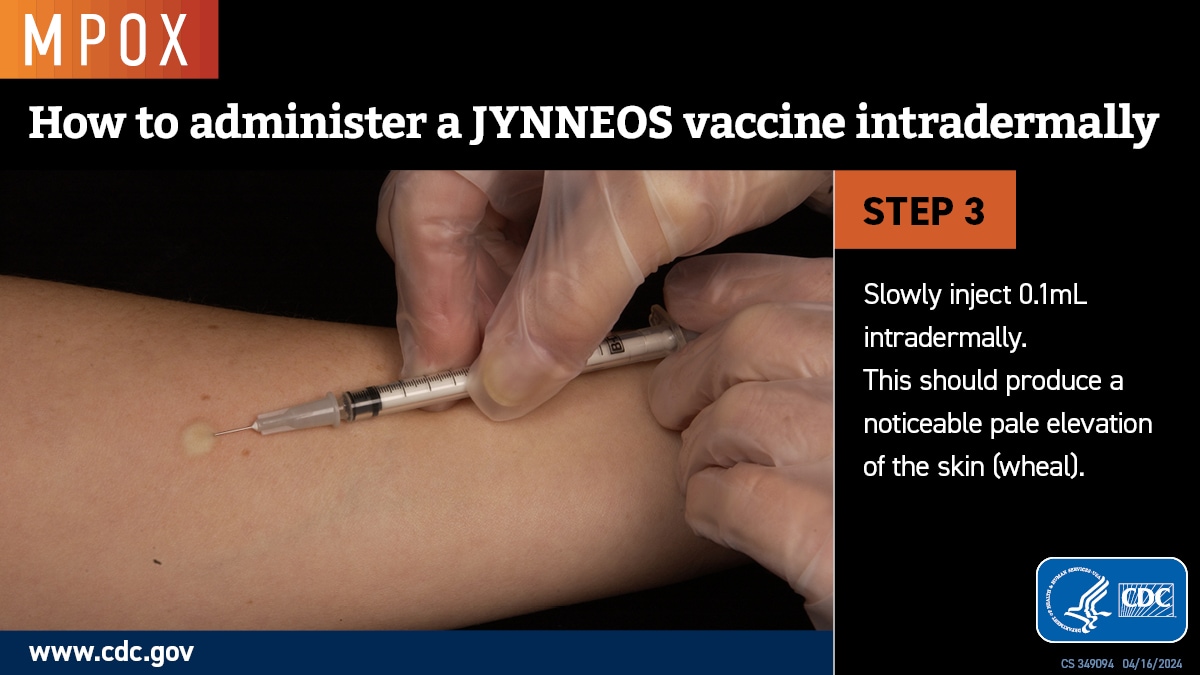
Intradermal administration involves injecting the vaccine superficially between the epidermis and the hypodermis layers of the skin, typically of the volar aspect (inner side) of the forearm. If the volar aspect of the forearm is not an option (e.g., strong patient preference), intradermal administration of vaccine may be performed at the upper back below the scapula or at the deltoid. Producing a noticeable pale elevation of the skin (wheal) with the intradermal injection is desirable but not required.
A person who presents for their second JYNNEOS vaccine dose who is still experiencing erythema or induration at the site of intradermal administration of the first vaccine dose (e.g., the forearm) should have the second dose administered intradermally in the contralateral forearm or if that is not an option, in the upper back below the scapula, or at the deltoid.
Video on Administering JYNNEOS Intradermally
How to Administer Intradermal Vaccine in Forearm, Deltoid, and Scapula
Video Length: 00:02:49
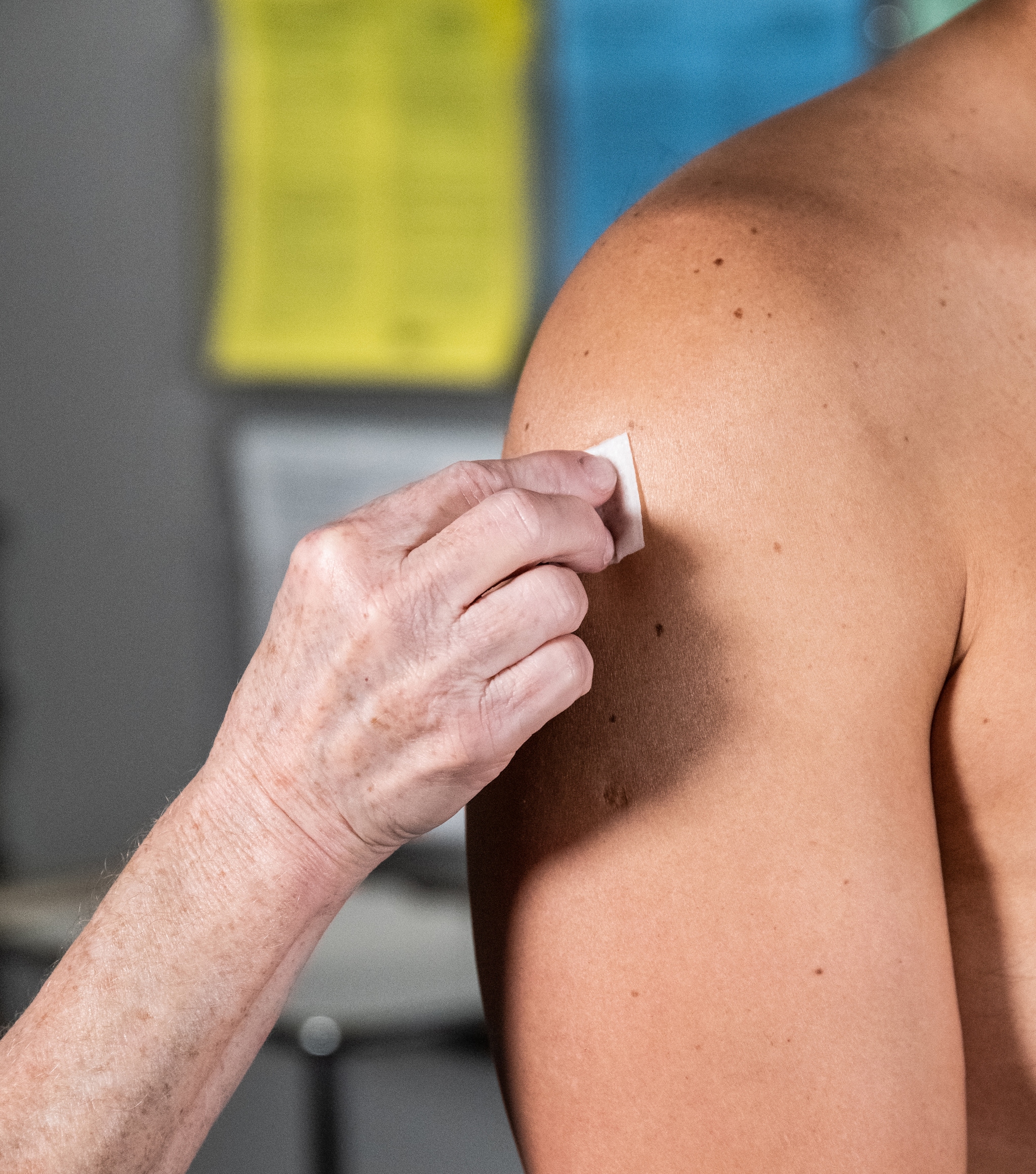
Example of locating and cleaning the site for intradermal administration at the deltoid.
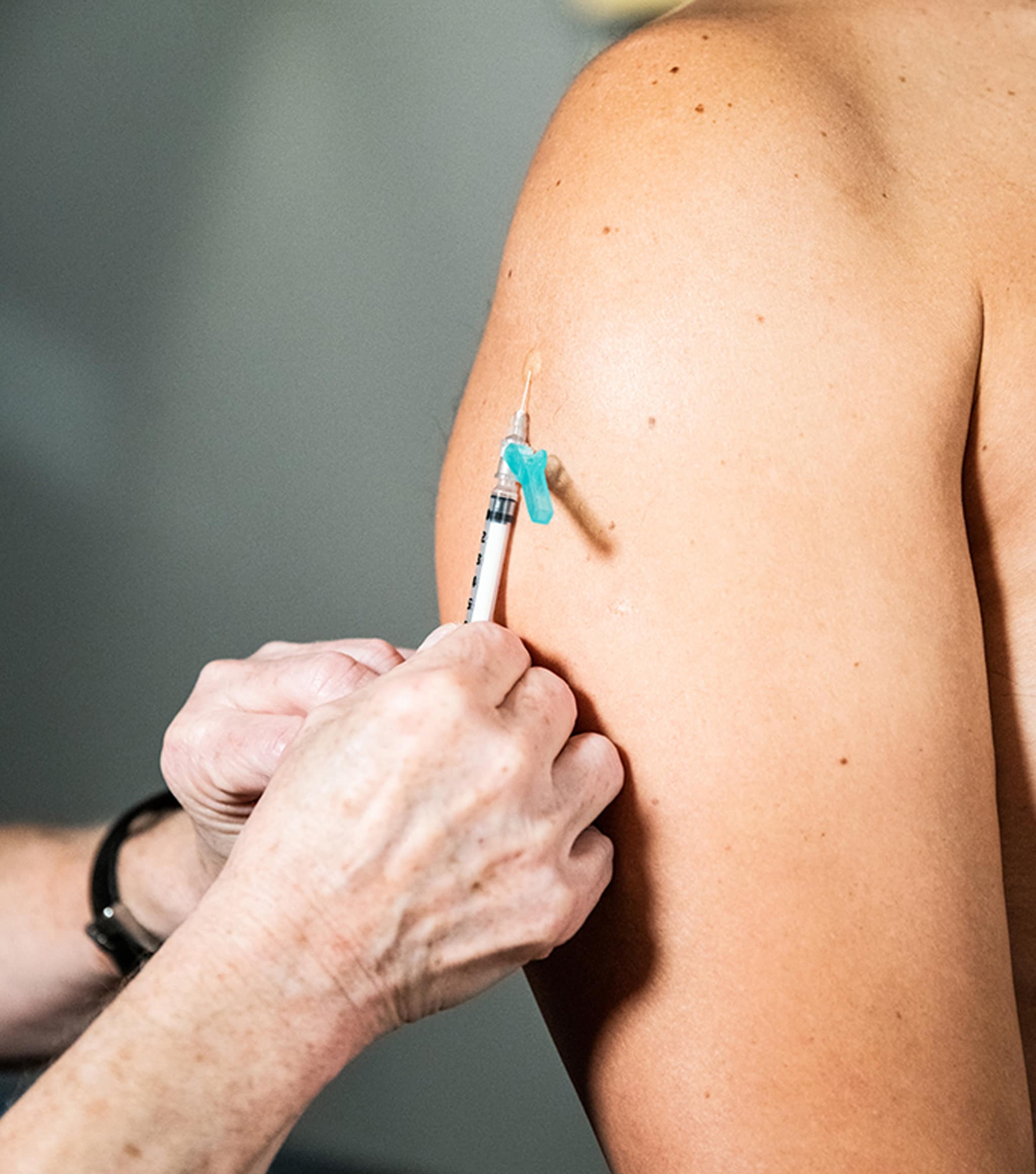
Example of intradermal administration at the deltoid.
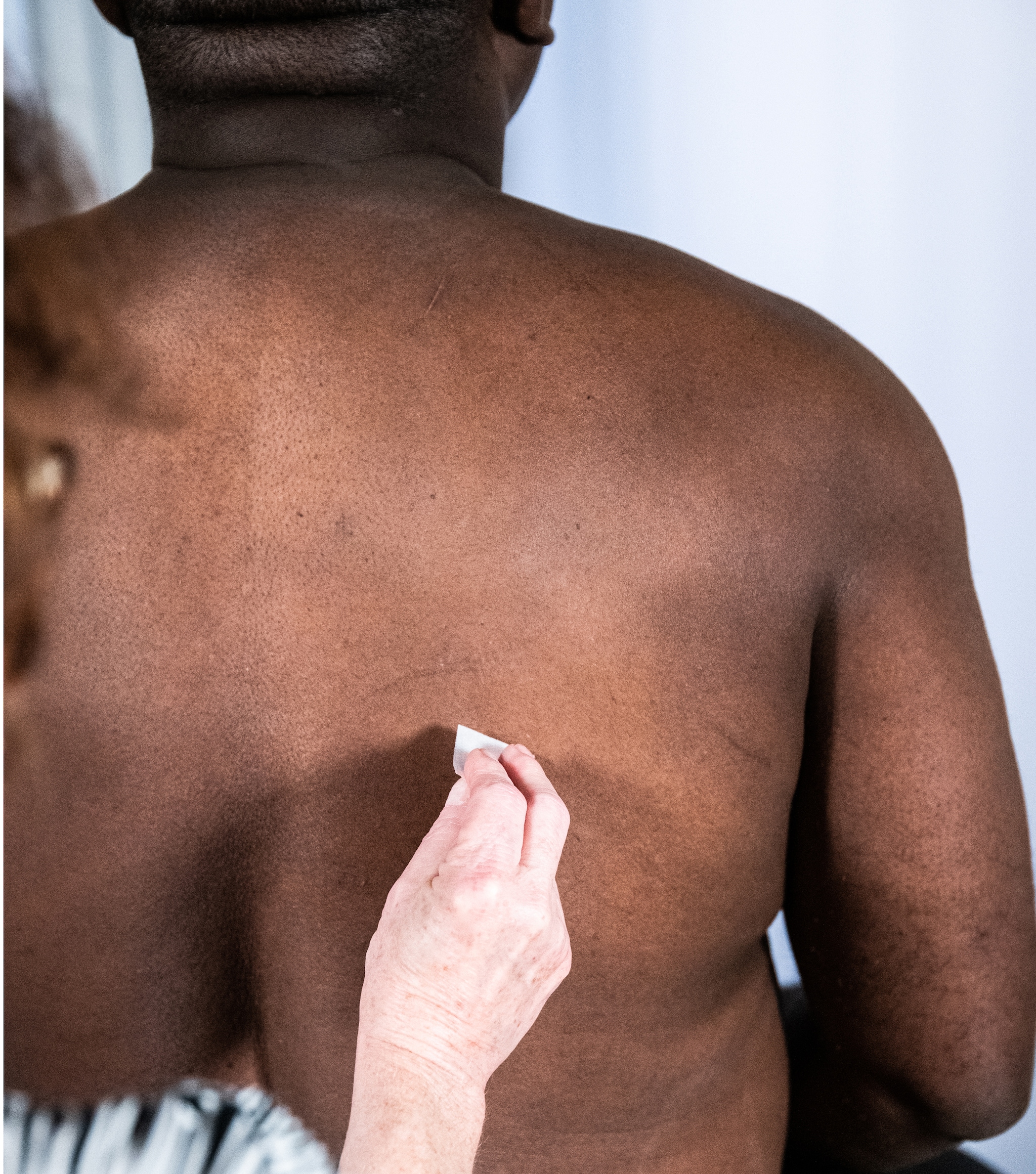
Example of locating and cleaning the site for intradermal administration at the upper back below the scapula.
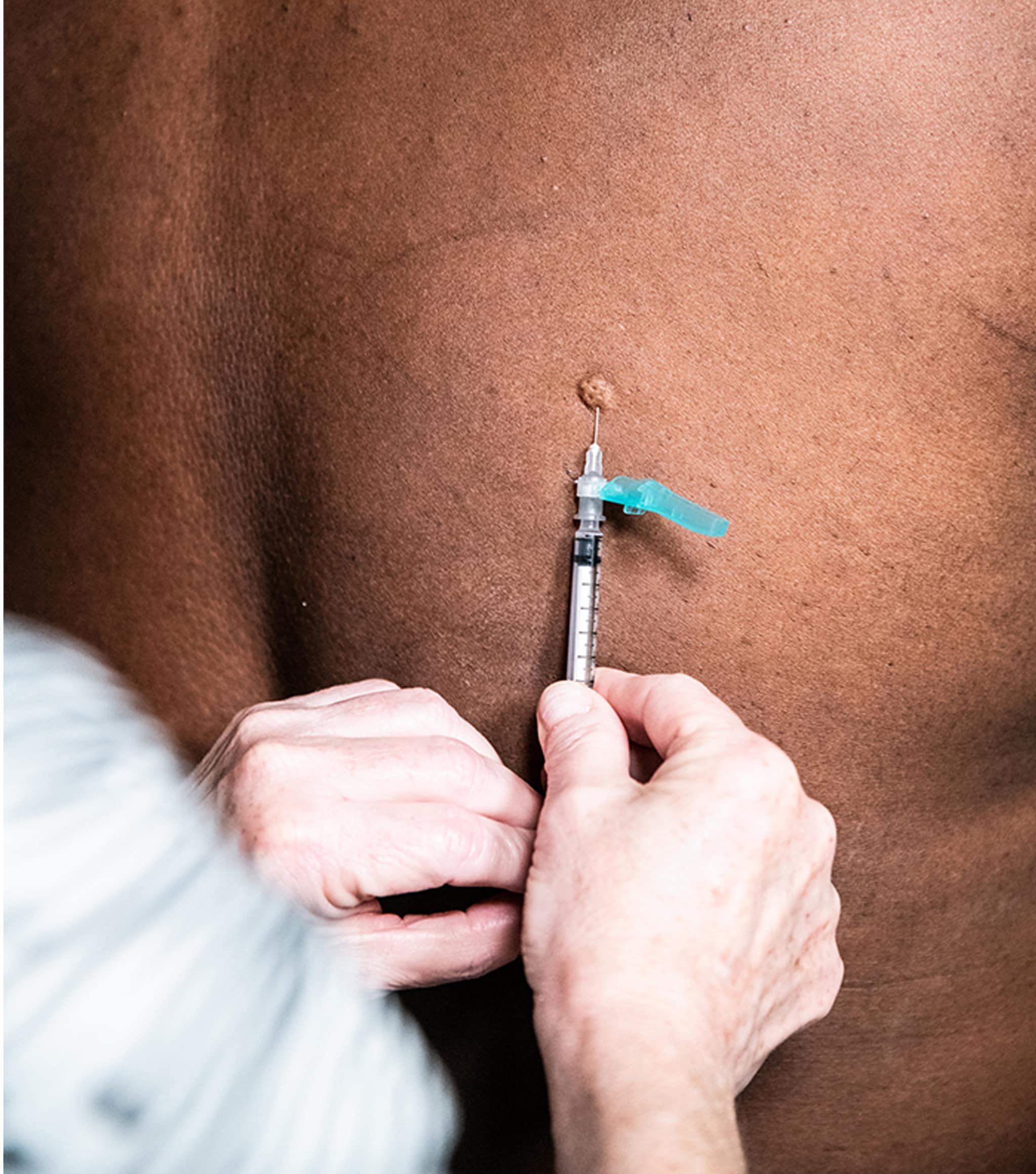
Example of intradermal administration at the upper back below the scapula.
Predrawing JYNNEOS Vaccine
Predrawing vaccines can result in waste if more are drawn up than needed. In addition, once vaccines are drawn into syringes, it is difficult to tell them apart, which can lead to administration errors. However, there may be rare instances when the only option is to predraw JYNNEOS vaccine. If vaccines must be predrawn:
- Predrawn vaccine must be labeled with vaccine name, lot number, date and time prepared, and preparer’s initials, and MUST BE refrigerated.
- Predrawn syringes must be stored at the manufacturer-recommended refrigerated temperatures throughout the clinic day.
- A separate clean administration station for each vaccine type should be set up to prevent medication errors.
- Vaccines should be drawn up into syringes only after arriving at the clinic site, or mass vaccination event. Drawing up doses days or even hours before administering them is not a best practice because general-use syringes are not designed for storage.
- Each person administering vaccines should draw up no more than 10 doses at one time.
- Patient flow should be monitored to avoid drawing up unnecessary doses.
- If a predrawn vaccine is not used within 8 hours of being drawn, the dose should be discarded.
- Predrawn vaccine should never be transferred back into a vial for storage.
Safe Injection Practices
Every year, unsafe injection practices by U.S. healthcare providers, such as syringe reuse and misuse of medications vials, causes outbreaks. It is the responsibility of every provider who prepares and administers injections, or supervises those that prepare and administer injections, to ensure that patients receive the correct medication and are not exposed to life-threatening infections. Providers should adhere to Standard Precautions and the principles of Safe Injection Practices, including the use of a sterile, single-use, disposable needle and syringe for each injection given, and prevention of contamination of injection equipment and medication.
See the following resources for further information, including on how to safely store, prepare, and administer vaccines:
- ACIP’s general best practices and Epidemiology and Prevention of Vaccine-Preventable Diseases (CDC Pink Book) and
- CDC’s Vaccine Administration Tools and
- CDC’s One and Only Campaign and FAQs regarding safe injection practices like preparation, administration, and handling of single-dose vials.
Coadministration of JYNNEOS vaccine with the tuberculin skin test
Currently, there are no data on administering JYNNEOS vaccine at the same time as the tuberculin skin test (TST). While JYNNEOS is a live virus vaccine, it is non-replicating and its effect on the response to the TST may not be the same as for live, replicating virus vaccines such as measles-mumps-rubella (MMR).
If a delay in the TST would cause substantial burden (e.g., preventing a person from working because of pre-employment screening policies) then the TST should not be delayed. If delays in the TST will not cause substantial burden, a delay of at least 4 weeks after JYNNEOS vaccination is preferred.
The TST can be performed at the same time as JYNNEOS vaccination and any sequence of vaccination and the TST may be used. If the JYNNEOS vaccine and the TST are administered on the same day, the vaccine and the TST should be administered on different forearms, one on the left and one on the right. The location of each injection site should be recorded in order to read the TST result from the correct forearm. If the JYNNEOS vaccine and the TST are administered on the same forearm, the sites of injection should be separated by 8–10 centimeters (that is, 3–4 inches) along the length of the forearm, to reduce likelihood of overlap of any reactions from the two injections, and the location of each injection site and the antigen should be recorded.
For patients who have symptoms or signs of active tuberculosis (TB), all tests and examinations for TB diagnosis should be pursued without delay, regardless of JYNNEOS vaccination.