Vaccine Administration
General Best Practices for Immunization
Printer friendly version [26 pages]
Infection Control and Sterile Technique
General Precautions
Persons administering vaccinations should follow appropriate precautions to minimize risk for disease exposure and spread. Hands should be cleansed with an alcohol-based waterless antiseptic hand rub or washed with soap and water before preparing vaccines for administration and between each patient contact (1). Occupational Safety and Health Administration (OSHA) regulations do not require gloves to be worn when administering vaccinations, unless persons administering vaccinations have open lesions on their hands or are likely to come into contact with a patient’s body fluids (2). If worn, gloves should be changed between patients.
Vaccine Administration: Preparation and Timely Disposal
Vaccines should be drawn up in a designated clean medication area that is not adjacent to areas where potentially contaminated items are placed. Multi-dose vials to be used for more than one patient should not be kept or accessed in the immediate patient treatment area. This is to prevent inadvertent contamination of the vial through direct or indirect contact with potentially contaminated surfaces or equipment that could then lead to infections in subsequent patients (3). Smallpox vaccine is accessed by dipping a bifurcated needle directly into the vaccine vial. The vaccine adheres to the sides of the bifurcated needle, and is administered via skin puncture. The vial must be accessed in the immediate patient area to reduce environmental contamination by vaccine virus. To prevent contamination of the vial, make sure the patient area is clean and free of potentially contaminated equipment.
Different single-components of combination vaccines should never be mixed in the same syringe by an end-user unless specifically licensed for such use (4). Single-dose vials and manufacturer-filled syringes are designed for single-dose administration and should be discarded if vaccine has been withdrawn or reconstituted and subsequently not used within the time frame specified by the manufacturer. Syringes that are prefilled by the manufacturer and activated (i.e., syringe cap removed or needle attached) but unused should be discarded at the end of the clinic day. For non-live vaccines, manufacturers typically recommend use within the same day that a vaccine is withdrawn or reconstituted. For live vaccines that require reconstitution, manufacturers typically recommend the vaccine be used as soon as possible after reconstitution and be discarded if not used within 30 minutes after reconstitution. For example, varicella vaccine should be discarded if not used within 30 minutes after reconstitution, whereas MMR vaccine, once reconstituted, must be kept in a dark place at 36°F to 46°F (2°C to 8°C) and should be discarded within 8 hours if not used. When in doubt about the appropriate handling of a vaccine, vaccination providers should contact that vaccine’s manufacturer.
ACIP discourages the routine practice of providers’ prefilling syringes for several reasons. Because the majority of vaccines have a similar appearance after being drawn into a syringe, prefilling might result in administration errors. Because unused prefilled syringes also typically must be discarded if not used within the same day that they are filled, vaccine wastage might occur. The FDA does not license administration syringes for vaccine storage.
In certain circumstances in which a single vaccine type is being used (e.g., in preparation for a community influenza vaccination campaign), filling a small number (10 or fewer) of syringes may be considered (5). The doses should be administered as soon as possible after filling, by the same person who filled the syringes. Unused syringes that are prefilled by the manufacturer and activated (i.e., syringe cap removed or needle attached) should be discarded at the end of the clinic day. Vaccine from two or more vials should never be combined to make one or more doses. This can lead to violation of expiration dates and product contamination (6,7).
Health Care Provider Exposure to Vaccine Components
Providers are sometimes concerned when they have the same contraindications or precautions as their patients from whom they withhold or defer vaccine. For administration of routinely recommended vaccines, there is no evidence of risk of exposure of vaccine components to the health care provider, so conditions in the provider labeled as contraindications and precautions to a vaccine components are not a reason to withdraw from this function of administering the vaccine to someone else. Historic concerns about exposure to vaccine components are limited to non-parenteral vaccines in which some degree of environmental exposure is unavoidable (5, 8), or situations in which self-inoculation is likely due to the nature of the vaccine microbe [e.g. reduced attenuation of smallpox vaccine virus (9)]. Persons administering ACAM 2000 smallpox vaccine to laboratory and health care personnel at risk for occupational exposure to orthopoxviruses can decrease the risk for inadvertent infection through recommended infection prevention measures. However, because of a theoretical risk for infection, vaccination with ACAM2000 can be offered to health care personnel administering this vaccine, provided individual persons have no specified contraindications to vaccination (10).
Safe Use of Needles and Syringes
Needles and syringes used for vaccine injections must be sterile and disposable. A separate needle and syringe should be used for each injection. Changing needles between drawing vaccine from a vial and injecting it into a recipient is not necessary unless the needle has been damaged or contaminated (11).
Bloodborne diseases (e.g., hepatitis B, hepatitis C, human immunodeficiency virus [HIV]) are occupational hazards for clinicians and other health-care providers. The Needlestick Safety and Prevention Act (2) was enacted in 2000 to reduce the incidence of needlestick injury and the consequent risk for bloodborne diseases acquired from patients. The act directed OSHA to strengthen its existing bloodborne pathogen standards. The revised standards became effective in 2001 (2). These federal regulations require the use of engineering and work practice controls to eliminate or minimize employee exposure to bloodborne pathogens. Engineering controls means controls (e.g., sharps disposal containers, self-sheathing needles, safer medical devices, such as sharps with engineered sharps injury protections and needleless systems) that isolate or remove the bloodborne pathogens hazard from the workplace). Needle-shielding or needle-free devices that might satisfy the occupational safety regulations for administering injectable vaccines are available in the United States (12-13). The regulations also require maintenance of records documenting injuries caused by needles and other medical sharp objects and that nonmanagerial employees be involved in the evaluation and selection of safety-engineered devices before they are procured. Additional information about implementation and enforcement of these regulations is available from OSHA.
To prevent inadvertent needlestick injury or reuse, safety mechanisms should be deployed after use and needles and syringes should be discarded immediately in labeled, puncture-proof containers located in the same room where the vaccine is administered (5). Used needles should never be recapped.
Route of Administration
Injectable Route
Routes of administration are recommended by the manufacturer for each immunobiologic (Table 6-1). With the exceptions of bacille Calmette-Guérin (BCG) vaccine and smallpox vaccine [ACAM2000] (both administered by the percutaneous route), injectable vaccines are administered by the intramuscular or subcutaneous route. The smallpox/monkeypox vaccine (Jynneos) is primarily administered by the subcutaneous route but in some circumstances is administered by the intradermal route. Deviation from the recommended route of administration might reduce vaccine efficacy (14-15) or increase the risk for local adverse reactions (16-18).
The method of administration of injectable vaccines is determined, in part, by the inclusion of adjuvants in some vaccines. An adjuvant is a vaccine component distinct from the antigen that enhances the immune response to the antigen, but might also increase risk of adverse reactions. To decrease risk of local adverse events, non-live vaccines containing an adjuvant should be injected into a muscle. Administering a vaccine containing an adjuvant either subcutaneously or intradermally can cause local irritation, induration, skin discoloration, inflammation, and granuloma formation.
Intramuscular Injections
Needle Length
Injectable immunobiologics should be administered where local, neural, vascular, or tissue injury is unlikely. Use of longer needles has been associated with less redness or swelling than occurs with shorter needles because of injection into deeper muscle mass (16). Appropriate needle length depends on age and body mass. Injection technique is the most important parameter to ensure efficient intramuscular vaccine delivery.
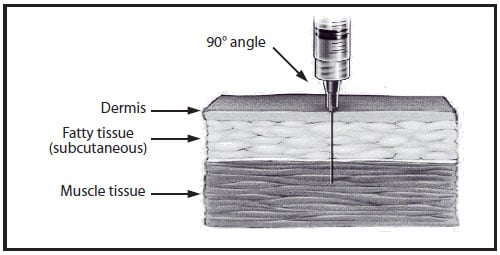
For all intramuscular injections, the needle should be long enough to reach the muscle mass and prevent vaccine from seeping into subcutaneous tissue, but not so long as to involve underlying nerves, blood vessels, or bone (15,19-22). Vaccinators should be familiar with the anatomy of the area into which they are injecting vaccine. Intramuscular injections are administered at a 90-degree angle to the skin, preferably into the anterolateral aspect of the thigh or the deltoid muscle of the upper arm, depending on the age of the patient (Table 6-2).
The needle gauge for intramuscular injection is 22-25 gauge. A decision on needle length and site of injection must be made for each person on the basis of the size of the muscle, the thickness of adipose tissue at the injection site, the volume of the material to be administered, injection technique, and the depth below the muscle surface into which the material is to be injected (Figure 1). Some experts allow intramuscular injection with a ⅝-inch needle but ONLY if the skin is stretched flat (21). If the subcutaneous and muscle tissue are bunched to minimize the chance of striking bone (19), a 1-inch needle or larger is required to ensure intramuscular administration. Aspiration before injection of vaccines or toxoids (i.e., pulling back on the syringe plunger after needle insertion but before injection) is not necessary because no large blood vessels are present at the recommended injection sites, and a process that includes aspiration might be more painful for infants (22).
Infants (Aged <12 Months)
For the majority of infants, the anterolateral aspect of the thigh is the recommended site for injection because it provides comparatively larger muscle mass than the deltoid (Figure 2) (23). In certain circumstances (e.g., physical obstruction to other sites and no reasonable indication to defer doses), the gluteal muscle can be used. If the gluteal muscle must be used, care should be taken to define the anatomic landmarks.(a) For the majority of infants, a 1-inch needle is sufficient to penetrate the thigh muscle.
Toddlers (Aged 12 Months-2 Years)
For toddlers, the anterolateral thigh muscle is preferred, and when this site is used, the needle should be at least 1 inch long. The deltoid muscle can be used if the muscle mass is adequate. If 2 vaccines are to be administered in a single limb, they should be spaced an inch apart (4, 24).
Children (Aged 3-10 Years)
The deltoid muscle is preferred for children aged 3-10 years (25); the needle length for deltoid site injections can range from ⅝ to 1 inch on the basis of technique. The anterolateral thigh can also be used (25). In this case the needle length should be 1 inch to 1.25 inches. Knowledge of body mass can be useful for estimating the appropriate needle length (26).
Young Adolescents (Aged 11-18 years)
The deltoid muscle is preferred for adolescents 11-18 years of age. The anterolateral thigh can also be used. For injection into the anterolateral thigh, most adolescents will require a 1-1.5-inch needle to ensure intramuscular administration (27).
Adults (Aged ≥19 Years)
For adults, the deltoid muscle is recommended for routine intramuscular vaccinations (23) (Figure 3). The anterolateral thigh also can be used. When injecting into the deltoid muscle, for adults a measurement of body mass/weight is allowable prior to vaccination, understanding that resources to measure body mass/weight are not available in all clinical settings. For men and women who weigh <130 lbs (<60 kg), a ⅝-inch needle is sufficient to ensure intramuscular injection in the deltoid muscle if the injection is made at a 90-degree angle and the tissue is not bunched. For men and women who weigh 130-152 lbs (60-70 kg), a 1-inch needle is sufficient. For women who weigh 152-200 lbs (70-90 kg) and men who weigh 152-260 lbs (70-118 kg), a 1- to 1.5-inch needle is recommended. For women who weigh >200 lbs (>90 kg) or men who weigh >260 lbs (>118 kg), a 1.5-inch needle is recommended (table 6-2) (20). For injection into the anterolateral thigh muscle, a 1.5-inch needle should be used, although a 1-inch needle may be used if the skin is stretched tightly and subcutaneous tissues are not bunched.
Subcutaneous Injections
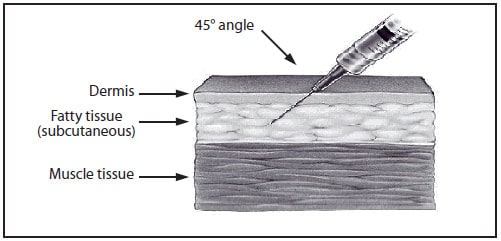
Subcutaneous injections are administered at a 45-degree angle, usually into the thigh for infants aged <12 months and in the upper-outer triceps area of persons aged ≥12 months. Subcutaneous injections may be administered into the upper-outer triceps area of an infant if necessary. A ⅝-inch, 23- to 25-gauge needle should be inserted into the subcutaneous tissue (Figures 4 and 5) (4).
Oral Route
Rotavirus, adenovirus, cholera vaccine, and oral typhoid vaccines are the only vaccines administered orally in the United States. Oral typhoid capsules should be administered as directed by the manufacturer. The capsules should not be opened or mixed with any other substance. Rotavirus vaccines are licensed for infants. There are 2 brands of rotavirus vaccine, and they have different types of applicators. Providers should consult package inserts for details.
Intranasal Route
Live attenuated influenza vaccine is approved for healthy nonpregnant persons aged 2-49 years and is the only vaccine administered by the intranasal route. The administration device is a nasal sprayer with a dose-divider clip that allows introduction of one 0.1-mL spray into each naris. The tip should be inserted slightly into the naris before administration. Even if the person coughs or sneezes immediately after administration or the dose is expelled any other way, the vaccine dose need not be repeated (5).
Severely immunosuppressed persons (i.e., those who require care in a protected environment, e.g., bone marrow transplant recipients, individuals with severe combined immunodeficiency diseases) should not administer LAIV. It would be uncommon for persons with these conditions to be in a role administering vaccines. Other persons at increased risk for influenza complications can administer LAIV. These include persons with underlying medical conditions placing them at higher risk or who are likely to be at risk, including pregnant women, persons with asthma, and persons aged ≥50 years (2).
Multiple Injections
If multiple vaccines are administered at a single visit, administer each preparation at a different anatomic site (28). The location of all injection sites with the corresponding vaccine injected should be documented in each patient’s medical record. Health-care practices should consider using a vaccination site map so that all persons administering vaccines routinely use a particular anatomic site for each particular vaccine.
For infants and younger children, if more than 2 vaccines are injected in a single limb, the thigh is the preferred site because of the greater muscle mass; the injections should be sufficiently separated (separate anatomic sites [i.e. ≥1 inch] if possible) so that any local reactions can be differentiated (13,29). For older children and adults, the deltoid muscle can be used for more than one intramuscular injection.
If a vaccine and an immune globulin preparation are administered simultaneously (e.g., Td/Tdap and tetanus immune globulin [TIG], hepatitis B and hepatitis B immunoglobulin [HBIG]), separate limbs should be used for each injection (29-30).
Jet Injections
Jet injectors are needle-free devices that pressurize liquid medication, forcing it through a nozzle orifice into a narrow stream capable of penetrating skin to deliver a drug or vaccine into intradermal, subcutaneous, or intramuscular tissues (32-33). Immune responses generated by jet injectors against both attenuated and non-live viral and bacterial antigens are usually equivalent to, and occasionally greater than, immune responses induced by needle injection. However, local reactions or injuries (e.g., skin laceration, transient neuropathy, hematoma) are sometimes more frequent on delivery of vaccine by jet injectors compared with needle injection, depending on the inherent irritability of the vaccine and operator technique (33).
Multiple use jet injectors using the same nozzle for consecutive injections without intervening sterilization were used in mass vaccination campaigns from the 1950s through the 1990s (33); however, these were found to be unsafe because of the possibility of bloodborne pathogen transmission (34-37) and should not be used. A new generation of jet injectors with disposable cartridges and syringes has been developed since the 1990s. With a new, sterile dose chamber and nozzle for each patient and correct use, these devices do not have the same safety concerns as multiple-use nozzle jet injectors. Several of the newer devices have been approved by FDA for use with specific vaccines (33). Jet injectors prevent needlestick injuries to health-care providers (2) and can overcome improper, unsterile reuse and other drawbacks of needles and syringes in developing countries (9, 38-39).
Methods for Alleviating Discomfort and Pain Associated with Vaccination
Comfort measures, such as distraction (e.g., playing music or pretending to blow away the pain), cooling of the injection site(s), topical analgesia, ingestion of sweet liquids, breastfeeding, swaddling, and slow, lateral swaying can help infants or children cope with the discomfort associated with vaccination (40-42). Pretreatment (30-60 minutes before injection) with a 5% topical lidocaine-prilocaine emulsion might decrease the pain of vaccination by causing superficial anesthesia (43-44). Evidence indicates that this cream does not interfere with the immune response to MMR (45). There is no evidence the cream interferes with other vaccines (46-49). Topical lidocaine-prilocaine emulsion should not be used on infants aged <12 months who are receiving treatment with methemoglobin-inducing agents (e.g., acetaminophen, amyl nitrate, nitroprusside, dapsone) because of the possible development of methemoglobinemia (50). Use of a topical refrigerant (vapocoolant) spray immediately before vaccination can reduce the short-term pain associated with injections and can be as effective as lidocaine-prilocaine cream (51). Evidence does not support use of antipyretics before or at the time of vaccination; however, they can be used for the treatment of fever and local discomfort that might occur following vaccination. Studies of children with previous febrile seizures have not demonstrated antipyretics to be effective in the prevention of febrile seizures (48).
Clinical Implications of Nonstandard Vaccination Practices
Best practice guidance for route, site, and dosage of immunobiologics is derived from data from clinical trials, practical experience, normal intervals of health care visits, and theoretical considerations. ACIP discourages variations from the recommended route, site, volume, or number of doses of any vaccine.
Variation from the recommended route and site can result in inadequate protection. In adults (but not in infants) (52), the immunogenicity of hepatitis B is substantially lower when the gluteal rather than the deltoid site is used for administration (8). Hepatitis B administered intradermally might result in a lower seroconversion rate and final titer of hepatitis B surface antibody than when administered by the deltoid intramuscular route (53-54). Hepatitis B administered by any route other than intramuscular, or in adults at any site other than the deltoid or anterolateral thigh, should not be counted as valid and should be repeated (9). Similarly, doses of rabies vaccine administered in the gluteal site should not be counted as valid doses and should be repeated (54). Hepatitis A vaccine and meningococcal conjugate vaccine do not need to be repeated if administered by the subcutaneous route (55-56). However, for DTaP, Hib, and PCV13, there is no evidence related to immunogenicity of these 3 vaccines given subcutaneously. Providers should address circumstances in which dose(s) of these vaccines have been administered subcutaneously on a case-by-case basis. Inactivated influenza vaccine is immunogenic when administered in a lower-than-standard dose by the intradermal route to healthy adult volunteers. Intradermal injection produced antibody responses similar to intramuscular injection in vaccinees aged 18-60 years (57). However, the immunogenicity for persons aged ≥65 years is inadequate, and varying the recommended route and dose either with the intradermal product licensed through 64 years of age or with other influenza vaccines is not recommended (24).
Live, attenuated injectable vaccines (e.g., MMR, varicella, yellow fever) and certain non-live vaccines (e.g., meningococcal polysaccharide) are recommended by the manufacturers to be administered by subcutaneous injection. PPSV23and IPV are recommended by the manufacturer to be administered by the subcutaneous or intramuscular route. Response to vaccines recommended by the subcutaneous route is unlikely to be affected if the vaccines are administered by the intramuscular rather than subcutaneous route. Repeating doses of vaccine administered by the intramuscular route when recommended to be by the subcutaneous route is not necessary (10).
Administering volumes smaller than recommended (e.g., inappropriately divided doses) might result in inadequate protection. Using reduced doses administered at multiple vaccination visits that equal a full dose or using smaller divided doses is not recommended (4). Any vaccination using less than the standard dose should not be counted, and the person should be revaccinated according to age unless serologic testing indicates that an adequate response has developed. However, if 2 half-volume formulations of vaccine have already been administered on the same clinic day to a patient recommended for the full volume formulation, these 2 doses can count as one full dose. If less than a full recommended dose of a vaccine is administered because of syringe, applicator, or needle leakage, the dose should be repeated (5). Using larger-than-recommended dosages can be hazardous because of excessive local or systemic concentrations of antigens or other vaccine constituents.
(a) If the gluteal muscle is chosen, injection should be administered lateral and superior to a line between the posterior superior iliac spine and the greater trochanter or in the ventrogluteal site, the center of a triangle bounded by the anterior superior iliac spine, the tubercle of the iliac crest, and the upper border of the greater trochanter.
TABLE 6-1. Dose and route of administration for selected vaccines
| Vaccine | Dose | Route |
|---|---|---|
| DEN4CYD | 0.5 mL | Subcut |
| DTaP, DT, Td, Tdap | 0.5 mL | IM |
| DTaP-HepB-IPV | 0.5 mL | IM |
| DTaP/Hib | 0.5 mL | IM |
| DTaP-IPV/Hib | 0.5 mL | IM |
| DTaP-IPV | 0.5 mL | IM |
| Hib | 0.5 mL | IM |
| Hib-MenCY | 0.5 mL | IM |
| HepA | ≤18 years: 0.5 mL ≥19 years: 1.0 mL |
IM |
| HepB | ≤19 years: 0.5 mL(a) ≥20 years: 1.0 mL |
IM |
| HepA-HepB | ≥18 years: 1.0 mL | IM |
| LAIV | 0.2 mL divided dose between nares | Intranasal spray |
| IIV | 6-35 months: 0.25 mL or 0.5 mL ≥3 years: 0.5 mL(b) |
IM |
| MenB | 0.5 mL | IM |
| MMR | 0.5 mL | Subcut |
| MMRV | 0.5 mL | Subcut |
| MenACWY | 0.5 mL | IM |
| MPSV4 | 0.5 mL | Subcut |
| PCV13 | 0.5 mL | IM |
| PCV15 | 0.5 mL | IM |
| PCV20 | 0.5 mL | IM |
| PPSV23 | 0.5 mL | IM or Subcut |
| HPV | 0.5 mL | IM |
| IPV | 0.5 mL | IM or Subcut |
| Rotavirus (RV1 or RV5) | (1.0 mL or 2.0 mL) | Oral |
| Varicella | 0.5 mL | Subcut |
| RZV | 0.5 mL(c) | IM |
Abbreviations: DEN4CYD = dengue vaccine; DT = diphtheria and tetanus toxoids; DTaP = diphtheria and tetanus toxoids and acellular pertussis; HepA = hepatitis A; HepB = hepatitis B; Hib = Haemophilus influenzae type b; HPV = human papillomavirus; IIV = inactivated influenza vaccine; IM = intramuscular; IPV = inactivated poliovirus; LAIV = live, attenuated influenza vaccine; MenACWY = quadrivalent meningococcal conjugate vaccine; MenB = serogroup B meningococcal vaccine; MenCY = bivalent meningococcal conjugate vaccine component; MMR = measles, mumps, and rubella; MMRV = measles, mumps, rubella, and varicella; MPSV4 = quadrivalent meningococcal polysaccharide vaccine; PCV13 = pneumococcal conjugate vaccine; PPSV23= pneumococcal polysaccharide vaccine; RV1 = live, attenuated monovalent rotavirus vaccine; RV5 = live, reassortment pentavalent rotavirus vaccine; RZV = recombinant adjuvanted zoster vaccine; Subcut = subcutaneous; Td = tetanus and diphtheria toxoids; Tdap = tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis.
Source: Adapted from Immunization Action Coalition.
(a) Persons aged 11-15 years may be administered Recombivax HB (Merck), 1.0 mL (adult formulation) on a 2-dose schedule.
(b) Note that prefilled syringes of High-Dose Fluzone have a volume of 0.7 cc and the recommended volume of administration is 0.7 ccs.
(c) Do not withdraw more than 0.5 mL from the reconstituted product, even if some product is left in the vial.
TABLE 6-2. Needle length and injection site of IM injections for children aged ≤18 years (by age) and adults aged ≥19 years (by sex and weight)
| Age group | Needle length | Injection site |
|---|---|---|
| Children (birth-18 years) | ||
| Neonates(a) | 5/8 inch (16 mm)(b) | Anterolateral thigh |
| Infants, 1-12 months | 1 inch (25 mm) | Anterolateral thigh |
| Toddlers, 1-2 years | 1-1.25 inch (25-32 mm) | Anterolateral thigh(c) |
| 5/8(b)-1 inch (16-25 mm) | Deltoid muscle of arm | |
| Children, 3-10 years | 5/8(b)-1 inch (16-25 mm) | Deltoid muscle of arm(c) |
| 1-1.25 inches (25-32 mm) | Anterolateral thigh | |
| Children, 11-18 years | 5/8(b)-1 inch (16-25 mm) | Deltoid muscle of arm(c) |
| 1-1.5 inches (25-38 mm) | Anterolateral thigh | |
| Adults (≥19 years) | ||
| Men and women, <60 kg (130 lbs) | 1 inch (25 mm)(d) | Deltoid muscle of arm |
| Men and women, 60-70 kg (130-152 lbs) | 1 inch (25 mm) | |
| Men, 70-118 kg (152-260 lbs) | 1-1.5 inches (25-38 mm) | |
| Women, 70-90 kg (152-200 lbs) | ||
| Men, >118 kg (260 lbs) | 1.5 inches (38 mm) | |
| Women, >90 kg (200 lbs) | ||
| Men and women, any weight | 1.5 inches (38 mm)(e) | Anterolateral thigh |
Abbreviation: IM = intramuscular.
Source: (17).
(a) First 28 days of life.
(b) If skin is stretched tightly and subcutaneous tissues are not bunched.
(c) Preferred site.
(d) Some experts recommend a 5/8-inch needle for men and women who weigh <60 kg, if used, skin must be stretched tightly (do not bunch subcutaneous tissue)
(e) Some experts recommend a 1-inch needle if the skin is stretched tightly and subcutaneous tissues are not bunched.
Figure 2. Intramuscular/subcutaneous site of administration: anterolateral thigh
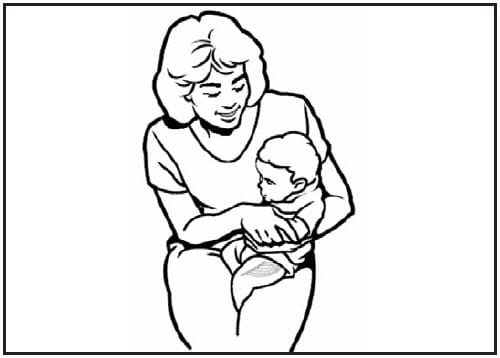
Source: Adapted from Minnesota Department of Health.
Figure 3. Intramuscular site of administration: deltoid
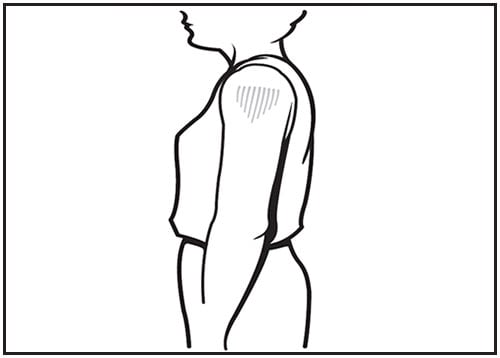
Source: Adapted from Minnesota Department of Health and Immunize.org.
Figure 4. Subcutaneous site of administration: triceps
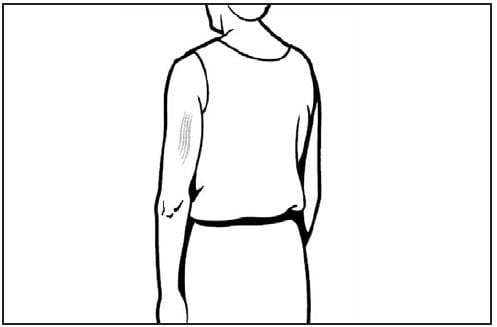
Source: Adapted from Minnesota Department of Health.
References
- Boyce JM, Pittet D. Guideline for hand hygiene in health-care settings. Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. MMWR Recomm Rep. 2002;51(RR-16):1-45, quiz CE41-44.
- Occupational Health and Safety Administration. Occupational exposure to bloodborne pathogens; needlesticks and other sharps injuries; Final Rule (29 CFR Part 1910). Fed Regist. 2001;66(12):5318-5325.
- Siegel J, Rhinehart E, Jackson M, Chiarello L, the Healthcare Infection Control Practices Advisory Committee. 2007 guideline for isolation precautions: preventing transmission of infectious agents in healthcare settings Atlanta, GA: CDC;2007.
- Centers for Disease Control and Prevention. General recommendations on immunization. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 1994;43(RR-1):1-38.
- Centers for Disease Control and Prevention. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009). MMWR. 2009;58(No. RR-8):1-54.
- Centers for Disease Control and Prevention. Recommendations for preventing transmission of infections among chronic hemodialysis patients. MMWR. 2001;50(No. RR-5):1-46.
- Atkinson WL, Pickering LK, Schwartz B, Weniger BG, Iskander JK, Watson JC. General recommendations on immunization. Recommendations of the Advisory Committee on Immunization Practices (ACIP) and the American Academy of Family Physicians (AAFP). MMWR Recomm Rep. 2002;51(RR- 2):1-35.
- Centers for Disease Control and Prevention. Prevention of Rotavirus Gastroenteritis Among Infants and Children: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. 2009;58(No. RR-2):1-26.
- Centers for Disease Control and Prevention. Recommendations for using smallpox vaccine in a prevent vaccination program: supplemental recommendations of the Advisory Committee on Immunization Practices (ACIP) and the Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR. 2003;52(No. RR-7):1-18).
- www.cdc.gov/mmwr/volumes/65/wr/pdfs/mm6510a2.pdf (accessed February 2, 2019).
- Kroger AT, Sumaya CV, Pickering LK, Atkinson WL. General recommendations on immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2011:1-60.
- Atkinson WL, Pickering LK, Schwartz B, Weniger BG, Iskander JK, Watson JC. General recommendations on immunization. Recommendations of the Advisory Committee on Immunization Practices (ACIP) and the American Academy of Family Physicians (AAFP). MMWR Recomm Rep. 2002;51(RR-2):1-35.
- Drucker E, Alcabes PG, Marx PA. The injection century: massive unsterile injections and the emergence of human pathogens. Lancet. 2001;358(9297):1989-1992. DOI: 10.1016/s0140-6736(01)06967-7
- International Health Care Worker Safety Center. List of safety-engineered sharp devices and other products designed to prevent occupational exposures to bloodborne pathogens. 2003. Accessed 07 Feb 2017.
- Shaw FE, Jr., Guess HA, Roets JM, et al. Effect of anatomic injection site, age and smoking on the immune response to hepatitis B vaccination. Vaccine. 1989;7(5):425-430. DOI: 10.1016/0264-410X(89)90157-6
- Zuckerman JN. The importance of injecting vaccines into muscle. Different patients need different needle sizes. BMJ. 2000;321(7271):1237-1238. DOI: 10.1136/bmj.321.7271.1237
- Ipp MM, Gold R, Goldbach M, et al. Adverse reactions to diphtheria, tetanus, pertussis-polio vaccination at 18 months of age: effect of injection site and needle length. Pediatrics. 1989;83(5):679-682.
- Michaels L, Poole RW. Injection granuloma of the buttock. Can Med Assoc J. 1970;102(6):626-628.
- Haramati N, Lorans R, Lutwin M, Kaleya RN. Injection granulomas. Intramuscle or intrafat? Arch Fam Med. 1994;3(2):146-148.
- Bergeson PS, Singer SA, Kaplan AM. Intramuscular injections in children. Pediatrics. 1982;70(6):944-948.
- Poland GA, Borrud A, Jacobson RM, et al. Determination of deltoid fat pad thickness. Implications for needle length in adult immunization. JAMA. 1997;277(21):1709-1711. DOI: 10.1001/jama.1997.03540450065037
- Groswasser J, Kahn A, Bouche B, Hanquinet S, Perlmuter N, Hessel L. Needle length and injection technique for efficient intramuscular vaccine delivery in infants and children evaluated through an ultrasonographic determination of subcutaneous and muscle layer thickness. Pediatrics. 1997;100(3 Pt 1):400-403. DOI: 10.1542/peds.100.3.400
- Ipp M, Taddio A, Sam J, Gladbach M, Parkin PC. Vaccine-related pain: randomised controlled trial of two injection techniques. Arch Dis Child. 2007;92(12):1105-1108. DOI: 10.1136/adc.2007.118695
- CDC. Recommendation of the Immunization Practices Advisory Committee: general recommendations on immunization MMWR Morb Mortal Wkly Rep. 1983;32(1):1-16.
- Kroger AT, Atkinson WL, Marcuse EK, Pickering LK. General recommendations on immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2006;55(RR-15):1-48.
- Jackson LA, Yu O, Nelson JC, et al. Injection site and risk of medically attended local reactions to acellular pertussis vaccine. Pediatrics. 2011;127(3):e581-587. DOI: 10.1542/peds.2010-1886
- Middleman AB, Anding R, Tung C. Effect of needle length when immunizing obese adolescents with hepatitis B vaccine. Pediatrics. 2010;125(3):e508-512. DOI: 10.1542/peds.2009-1592
- Fiore AE, Uyeki TM, Broder K, et al. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep. 2010;59(RR-8):1-62.
- CDC. General recommendations on immunization: recommendations of the Public Health Service Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 1976;25(44):1-3.
- Scheifele D, Bjornson G, Barreto L, Meekison W, Guasparini R. Controlled trial of Haemophilus influenzae type B diphtheria toxoid conjugate combined with diphtheria, tetanus and pertussis vaccines, in 18-month-old children, including comparison of arm versus thigh injection. Vaccine. 1992;10(7):455-460. DOI: 10.1016/0264-410X(92)90394-Y
- CDC. Diphtheria, tetanus, and pertussis: recommendations for vaccine use and other preventive measures. Recommendations of the Immunization Practices Advisory Committee (ACIP). MMWR Recomm Rep. 1991;40(RR-10):1-28.
- Mast EE, Margolis HS, Fiore AE, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) part 1: immunization of infants, children, and adolescents. MMWR Recomm Rep. 2005;54(RR-16):1-31.
- Hingson RA, Davis HS, Rosen M. Historical development of jet injection and envisioned uses in mass immunization and mass therapy based upon 2 decades experience. Mil Med. 1963;128(6):516-524.
- Weniger B, Papania M. Alternative vaccine delivery methods. In: Plotkin S, Orenstein W, Offit P, eds. Vaccines. 5th ed. China: Saunders/Elsevier; 2008:1357-1392.
- CDC. Hepatitis B associated with jet gun injection—California. MMWR Morb Mortal Wkly Rep. 1986;35(23):373-376.
- Canter J, Mackey K, Good LS, et al. An outbreak of hepatitis B associated with jet injections in a weight reduction clinic. Arch Intern Med. 1990;150(9):1923-1927. DOI: 10.1001/archinte.1990.00390200105020
- Hoffman PN, Abuknesha RA, Andrews NJ, Samuel D, Lloyd JS. A model to assess the infection potential of jet injectors used in mass immunisation. Vaccine. 2001;19(28-29):4020-4027. DOI: 10.1016/S0264-410X(01)00106-2
- Kelly K, Loskutov A, Zehrung D, et al. Preventing contamination between injections with multiple-use nozzle needle-free injectors: a safety trial. Vaccine. 2008;26(10):1344-1352. DOI: 10.1016/j.vaccine.2007.12.041
- Simonsen L, Kane A, Lloyd J, Zaffran M, Kane M. Unsafe injections in the developing world and transmission of bloodborne pathogens: a review. Bull World Health Organ. 1999;77(10):789-800.
- Kane A, Lloyd J, Zaffran M, Simonsen L, Kane M. Transmission of hepatitis B, hepatitis C and human immunodeficiency viruses through unsafe injections in the developing world: model-based regional estimates. Bull World Health Organ. 1999;77(10):801-807.
- Gray L, Watt L, Blass EM. Skin-to-skin contact is analgesic in healthy newborns. Pediatrics. 2000;105(1):e14. DOI: 10.1542/peds.105.1.e14
- Gray L, Miller LW, Philipp BL, Blass EM. Breastfeeding is analgesic in healthy newborns. Pediatrics. 2002;109(4):590-593. DOI: 10.1542/peds.109.4.590
- Harrington JW, Logan S, Harwell C, et al. Effective analgesia using physical interventions for infant immunizations. Pediatrics. 2012;129(5):815-822. DOI: 10.1542/peds.2011-1607
- Taddio A, Nulman I, Goldbach M, Ipp M, Koren G. Use of lidocaine-prilocaine cream for vaccination pain in infants. J Pediatr. 1994;124(4):643-648. DOI: 10.1016/S0022-3476(05)83150-6
- Uhari M. A eutectic mixture of lidocaine and prilocaine for alleviating vaccination pain in infants. Pediatrics. 1993;92(5):719-721.
- Gupta NK, Upadhyay A, Dwivedi AK, Agarwal A, Jaiswal V, Singh A. Randomized controlled trial of topical EMLA and vapocoolant spray for reducing pain during wDPT vaccination. World J Pediatr. 2017;13(3):236-241.
- Gupta NK, Upadhyay A, Agarwal A, Goswami G, Kumar J, Sreenivas V. Randomized controlled trial of topical EMLA and breastfeeding for reducing pain during wDPT vaccination. Eur J Pediatr. 2013;172:1527-1533.
- Reis EC, Holubkov R. Vapocoolant spray is equally effective as EMLA cream in reducing immunization pain in school-aged children. Pediatrics. 1997;100(6):E5. DOI: 10.1542/peds.100.6.e5
- Jacobsen RM, Swan A, Adegbenro A, et. al. Making vaccines more acceptable – methods to prevent and minimize pain and other common adverse events associated with vaccines. Vaccine. 2001;19:2418-2427.
- Halperin SA, McGrath P, Smith B, Houston T. Lidocaine-prilocaine patch decreases the pain associated with the subcutaneous administration of measles-mumps-rubella vaccine but does not adversely affect the antibody response. J Pediatr. 2000;136(6):789-794. DOI: 10.1016/S0022-3476(00)64169-0
- Frayling IM, Addison GM, Chattergee K, Meakin G. Methaemoglobinaemia in children treated with prilocaine-lignocaine cream. BMJ. 1990;301(6744):153-154. DOI: 10.1136/bmj.301.6744.153
- American Academy of Pediatrics Steering Committee on Quality Improvement and Management, Subcommittee on Febrile Seizures. Febrile seizures: clinical practice guideline for the long-term management of the child with simple febrile seizures. Pediatrics. 2008;121(6):1281-1286. DOI: 10.1542/peds.2008-0939
- Cook IF, Murtagh J. Comparative immunogenicity of hepatitis B vaccine administered into the ventrogluteal area and anterolateral thigh in infants. J Paediatr Child Health. 2002;38(4):393-396. DOI: 10.1046/j.1440-1754.2002.00013.x
- Redfield RR, Innis BL, Scott RM, Cannon HG, Bancroft WH. Clinical evaluation of low-dose intradermally administered hepatitis B virus vaccine. A cost reduction strategy. JAMA. 1985;254(22):3203-3206. DOI: 10.1001/jama.1985.03360220069031
- Coleman PJ, Shaw FE, Jr., Serovich J, Hadler SC, Margolis HS. Intradermal hepatitis B vaccination in a large hospital employee population. Vaccine. 1991;9(10):723-727. DOI: 10.1016/0264-410X(91)90287-G
- Fishbein DB, Sawyer LA, Reid-Sanden FL, Weir EH. Administration of human diploid-cell rabies vaccine in the gluteal area. N Engl J Med. 1988;318(2):124-125. DOI: 10.1056/nejm198801143180219
- CDC. Inadvertent misadministration of meningococcal conjugate vaccine—United States, June-August 2005. MMWR Morb Mortal Wkly Rep. 2006;55(37):1016-1017.
- Ragni MV, Lusher JM, Koerper MA, Manco-Johnson M, Krause DS. Safety and immunogenicity of subcutaneous hepatitis A vaccine in children with haemophilia. Haemophilia. 2000;6(2):98-103. DOI: 10.1046/j.1365-2516.2000.00386.x
- Belshe RB, Newman FK, Cannon J, et al. Serum antibody responses after intradermal vaccination against influenza. N Engl J Med. 2004;351(22):2286-2294. DOI: 10.1056/NEJMoa043555