Update on Immunodeficiency-Associated Vaccine-Derived Polioviruses — Worldwide, July 2018–December 2019
Weekly / July 17, 2020 / 69(28);913–917
Grace Macklin, MSc1; Ousmane M. Diop, PhD1; Asghar Humayun, MD2; Shohreh Shahmahmoodi, PhD3; Zeinab A. El-Sayed, MD, PhD4; Henda Triki, MD5; Gloria Rey, MSc6; Tigran Avagyan, MSc9; Varja Grabovac, MSc9; Jaume Jorba, PhD7; Noha Farag, MD, PhD8; Ondrej Mach, MD1 (View author affiliations)
View suggested citationSummary
What is already known about this topic?
Immunodeficiency-associated vaccine-derived polioviruses (iVDPVs) emerge among persons with primary immunodeficiencies (PIDs) and rarely can persist. Persistent iVDPV infection can result in paralysis and potentially seed community transmission.
What is added by this report?
After the 2016 global removal of oral poliovirus vaccine type 2 from routine immunization, the reported incidence of iVDPV type 2 infections markedly declined. Increasing surveillance among patients with PID has identified more iVDPV infections without paralysis.
What are the implications for public health practice?
Surveillance for iVDPV infections among patients with PID needs to be strengthened, and development of poliovirus antivirals needs to be accelerated to treat iVDPV infections to achieve and maintain eradication of all polioviruses.
Since establishment of the Global Polio Eradication Initiative* in 1988, polio cases have declined >99.9% worldwide; extensive use of live, attenuated oral poliovirus vaccine (OPV) in routine childhood immunization programs and mass campaigns has led to eradication of two of the three wild poliovirus (WPV) serotypes (types 2 and 3) (1). Despite its safety record, OPV can lead to rare emergence of vaccine-derived polioviruses (VDPVs) when there is prolonged circulation or replication of the vaccine virus. In areas with inadequate OPV coverage, circulating VDPVs (cVDPVs) that have reverted to neurovirulence can cause outbreaks of paralytic polio (2). Immunodeficiency-associated VDPVs (iVDPVs) are isolated from persons with primary immunodeficiency (PID). Infection with iVDPV can progress to paralysis or death of patients with PID, and excretion risks seeding cVDPV outbreaks; both risks might be reduced through antiviral treatment, which is currently under development. This report updates previous reports and includes details of iVDPV cases detected during July 2018–December 2019 (3). During this time, 16 new iVDPV cases were reported from five countries (Argentina, Egypt, Iran, Philippines, and Tunisia). Alongside acute flaccid paralysis (AFP) surveillance (4), surveillance for poliovirus infections among patients with PID has identified an increased number of persons excreting iVDPVs (5). Expansion of PID surveillance will facilitate early detection and follow-up of iVDPV excretion among patients with PID to mitigate the risk for iVDPV spread. This will be critical to help identify all poliovirus excretors and thus achieve and maintain eradication of all polioviruses.
Classification of VDPVs and Identification of iVDPV
Poliovirus isolates are grouped into three categories: WPV, Sabin-related poliovirus, and VDPV (3). Sabin-related viruses have limited divergence in the capsid protein (VP1) nucleotide sequences from the corresponding OPV (Sabin) strain: poliovirus types 1 and 3 (PV1 and PV3) are ≤1% divergent; poliovirus type 2 (PV2) is ≤0.6% divergent. VDPVs have clinical characteristics similar to those of WPV. VDPVs are >1% divergent (from PV1 and PV3) or >0.6% divergent (from PV2) in VP1 nucleotide sequences from the corresponding OPV strain (4). VDPVs are further classified as 1) circulating vaccine-derived polioviruses (cVDPVs), when there is evidence of community transmission; 2) iVDPVs, when they are isolated from persons with PIDs; and 3) ambiguous VDPVs (aVDPVs), when isolated from persons with no known immunodeficiency and when there is no evidence of community transmission or when isolates from sewage are not genetically linked to other known VDPVs and whose source is unknown (3).
A healthy person typically clears poliovirus infection within 6 weeks. However, in persons with PIDs, an inability to mount an adequate humoral immune response can result in persistence of intestinal infection with poliovirus and prolonged viral shedding (5,6). The iVDPV case definition is a laboratory-confirmed VDPV infection in a person of any age who has a primary humoral (B-cell) or combined humoral and cellular (B- and T-cell) immunodeficiency disorder (6). An iVDPV infection is persistent if VDPV is excreted for >6 months and chronic if excreted for >5 years (6).
Summary of iVDPV Epidemiology, 1961–2019
The World Health Organization (WHO) has compiled reports of iVDPV excretion since 1961 (6). As of May 2020, a total of 149 iVDPV cases had been reported to WHO from January 1961 through December 2019 (Table 1). These cases were detected through AFP surveillance (when paralysis occurred before PID was diagnosed) and by reports of iVDPV isolation from fecal specimens (when stool cultures were obtained from patients with suspected or diagnosed PID to detect enterovirus infection). The number of reported cases has increased over time: 66% of cases were detected during 2010–2019. Most onsets occurred in children aged <2 years (59%); 60% of cases were in males; and 64% of patients had acute flaccid paralysis (AFP) as the first sign. The most common PID diagnoses were various antibody disorders, severe combined immunodeficiency disorder (SCID), and common variable immunodeficiency disorder.
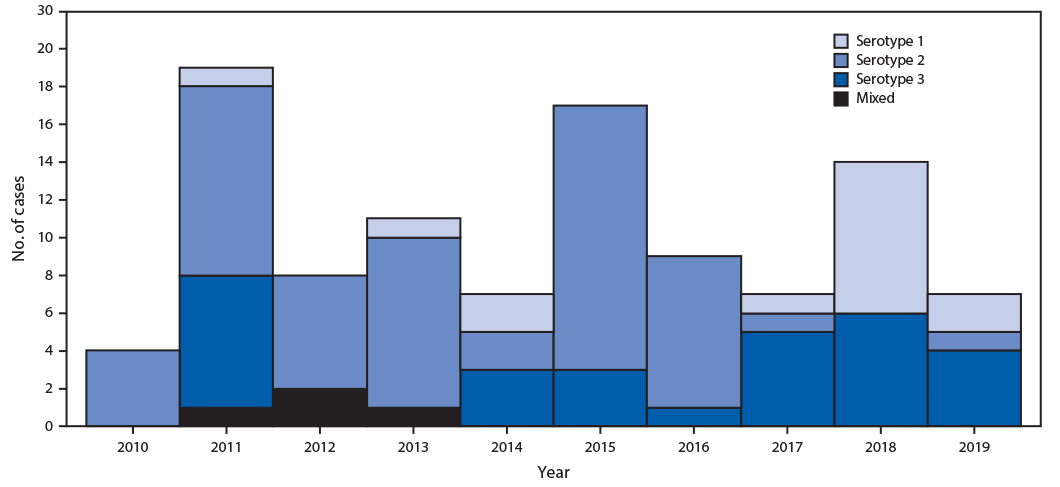
During the reporting period, iVDPV type 2 (iVDPV2) has been the most prevalent serotype (56%), followed by iVDPV type 3 (iVDPV3) (23%) and iVDPV type 1 (iVDPV1) (17%), with 4% heterotypic mixtures (types 1 and 2 in 2% of cases and types 2 and 3 in 2%). Because WPV type 2 had been eradicated, in April 2016, all 155 OPV-using countries and territories switched from trivalent OPV (tOPV, containing types 1, 2, and 3 Sabin strains) to bivalent OPV (bOPV, containing types 1 and 3 Sabin strains), to reduce the risk for paralytic disease from type 2 OPV (from vaccine-associated paralytic polio, which rarely occurs in OPV recipients and their susceptible close contacts; and from VDPV) (7). Since the tOPV-to-bOPV switch, the incidence of iVDPV2 cases has declined substantially, with iVDPV1 and iVDPV3 now the most prevalent serotypes (Figure). During 2000–2016, an average of 7.7 cases of iVDPV2 were identified per year (total = 54), compared with 0.67 cases per year (two cases) during 2017–2019. At the most recent follow-up, 16 patients (11%) were alive and still excreting iVDPV, 52 (35%) were alive and had stopped excreting, 65 (44%) had died, and 16 (11%) were lost to follow up (Table 1).
Reported iVDPV Cases, July 1, 2018–December 31, 2019
During July 2018–December 2019, 16 new iVDPV cases were reported from five countries (Argentina, Egypt, Iran, Philippines, and Tunisia) (Table 2). These cases included eight iVDPV1 cases, seven iVDPV3 cases, and one iVDPV2 case, with no heterotypic mixtures. The cases are described below.
Argentina (one case). In 2018 AFP occurred in a girl aged 9 months who had previously received 2 inactivated poliovirus vaccine doses and 1 bOPV dose in November 2017. In November 2018, iVDPV3 (1.4% VP1 divergence) was detected in a stool specimen. The most recent detection (2.9% VP1 divergence) was collected in August 2019; specimens collected since have been negative, the latest in November 2019. This patient had a diagnosis of agammaglobulinemia.
Egypt (10 cases). During July–December 2018, the PID surveillance project in Egypt identified six iVDPV infections, one in a patient who had developed AFP; two cases were iVDPV3 and four iVDPV1. Follow-up revealed that three patients had died, two patients stopped shedding, and one patient shedding iVDPV1 with 2.6% VP1 divergence continued to shed the virus for 22 months after the last reported bOPV dose. During 2019, four patients with iVDPV infection without AFP were detected; three patients had positive test results for iVDPV3, and one patient had a positive test result for iVDPV1. Two patients with iVDPV3 infection stopped excreting after 4 and 6 months.
Iran (three cases). In 2018, three iVDPV1 cases were reported, including a case detected before July 2018 and previously reported. These included cases in a boy aged 8 months with SCID who subsequently died and another in a boy aged 11 months who developed AFP in November 2018 and is continuing to excrete, most recently in April 2020. In July 2019, an iVDPV1 case was reported in a girl aged 7 months who had developed AFP; all seven specimens obtained from this patient contained iVDPV1.
Philippines (one case). An iVDPV2 case was detected in August 2019 in a boy aged 5 years who had received 3 doses of tOPV from 2014 to 2015. At his initial evaluation, he had severe malnutrition, significantly reduced antibody levels, and multiple signs and symptoms pointing to a complex immune disorder; however, no specific PID diagnosis was reported. Follow-up stool specimens collected from September 2019 to May 2020 were positive for VDPV2. Concurrent with the detection of the iVDPV2, a cVDPV2 outbreak was detected in the Philippines (2). Current genetic evidence indicates that the virus in the patient with iVDPV2 and cases in the cVDPV2 outbreak have similar genetic distance from parental OPV2 strain (7% VP1 divergence) and might share a common origin.
Tunisia (one case). A boy aged 9 months with human leukocyte antigen (HLA)-class II deficiency developed AFP in March 2019. The infant had previously received inactivated poliovirus vaccine and had no history of OPV vaccination. VDPV3 with 1.3%–4.1% VP1 divergence was detected in stool specimens collected during March–December 2019. The child had stopped excreting by March 2020.
Discussion
Most countries with AFP surveillance detect iVDPV in paralyzed children who then receive a diagnosis of one of the PIDs. However, many iVDPV cases occur in patients with PID without paralysis, and at present, are only detected through special studies or pilot projects of iVDPV surveillance in children with a diagnosed PID. The increase in the number of reported infections during 2010–2019 is likely a consequence of increased efforts to identify infection among patients with PID and improved methods to detect polioviruses. One half of the detected cases were from the WHO Eastern Mediterranean Region, likely related to more recent focus on PID surveillance in that region as well as higher rates of consanguineous marriages, which lead to higher prevalence rates of PID (8). WHO has supported several countries in implementing pilot projects for iVDPV surveillance in children with PID, including Egypt, Iran, Pakistan, Sri Lanka, and Tunisia, and more recently, China and India. Additional countries are being identified in other WHO regions and encouraged to implement systematic surveillance in children with PID and without paralysis. WHO and partners have developed guidelines for iVDPV surveillance in patients with PID that should become an integral part of global poliovirus surveillance (9).
Detection of cVDPV2 in the Philippines was associated with detection of VDPV2 infection in an immunodeficient patient. This is the first time that an iVDPV and cVDPV linkage has been described in a large outbreak, and further genetic analysis is in progress. It is, however, unclear how or whether the immunodeficient patient contributed to the cVDPV outbreak. The first identified poliovirus of the cVDPV2 outbreak was detected through environmental surveillance with 7% VP1 divergence from parental Sabin type 2 OPV and multiple amino acid changes. The cVDPV2 outbreak was confirmed by isolation of genetically linked virus from multiple additional sewage samples and AFP cases. A higher proportion of nucleotide substitutions leading to amino acid changes is usually found in genomic sequences of identified iVDPV2 from patients with PID.
Continued progress in the development of antiviral medications effective against polioviruses is needed to eliminate virus shedding in persons identified with persistent and chronic iVDPV infections. Pocapavir (a capsid inhibitor) has been administered on compassionate use basis for several patients excreting iVDPV, with mixed results (10). Complete clearing of virus has been observed in some recipients; however, rapid development of poliovirus resistance to Pocapavir has been frequently observed (10). Therefore, development of a treatment combining Pocapavir with a protease inhibitor currently called V-7404 that is expected to avoid antiviral resistance is continuing. Intravenous immunoglobulin is available to treat patients with PID and poliovirus (as well as nonpolio enterovirus) infection. While antiviral development continues, intravenous immunoglobulin might improve clinical care. Expansion of PID surveillance will facilitate early detection and follow-up of iVDPV excretion among patients with PID to mitigate the risk for iVDPV spread. This will be critical to help identify all poliovirus excretors and thus achieve and maintain eradication of all polioviruses.
Acknowledgments
Global Polio Laboratory Network; Eugene Saxentoff, World Health Organization (WHO) Regional Office, Copenhagen, Denmark; Nicksy Gumede, WHO Regional Office, Brazzaville, Republic of Congo; Ana Chevaz, WHO Regional Office, Washington, DC; Sirima Pattamadilok, WHO Regional Office, New Delhi, India; Mohamed A Sibak, WHO Regional Office, Cairo, Egypt; Steven Wassilak, Global Immunization Division, Center for Global Health, CDC; Qi Chen, Chadi Agha, Beth Henderson, Hongmei Liu, Kun Zhao, Jane Iber, Cara C. Burns, M. Steven Oberste, Division of Viral Diseases, National Center for Immunization and Respiratory Diseases, CDC; Shaza Badr, Ministry of Health and Population, Cairo, Egypt; Laila Bassiouni, Regional Reference Polio Laboratory, Cairo, Egypt; Elham M Hossny, Pediatric Allergy and Immunology Unit, Children’s Hospital, Ain Shams University, Cairo, Egypt; Nermeen M Galal; Pediatric Department, Cairo University, Cairo, Egypt; Ihab El-Sawy, Pediatric Respiratory Allergy and Immunology Unit, Alexandria University, Egypt.
Corresponding author: Ondrej Mach, macho@who.int, 41-22-791-1863.
1World Health Organization, Geneva, Switzerland; 2World Health Organization Regional Office, Cairo, Egypt; 3Tehran University of Medical Sciences, Tehran, Iran; 4Pediatric Allergy and Immunology Unit, Children’s Hospital, Ain Shams University, Cairo, Egypt; 5Laboratory of Clinical Virology, World Health Organization Reference Laboratory on Poliomyelitis, Institut Pasteur de Tunis, Tunisia; 6World Health Organization Regional Office, Washington, DC; 7Division of Viral Diseases, National Center for Immunization and Respiratory Diseases, CDC; 8Global Immunization Division, Center for Global Health, CDC; 9World Health Organization Regional Office, Manila, Philippines.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflicts of interest were disclosed.
References
- Chard AN, Datta SD, Tallis G, et al. Progress toward polio eradication—worldwide, January 2018–March 2020. MMWR Morb Mortal Wkly Rep 2020;69:784–9. CrossRef PubMed
- Alleman MM, Jorba J, Greene SA, et al. Update on vaccine-derived poliovirus outbreaks—worldwide, July 2019–February 2020. MMWR Morb Mortal Wkly Rep 2020;69:489–95. CrossRef PubMed
- Jorba J, Diop OM, Iber J, et al. Update on vaccine-derived polioviruses—worldwide, January 2017–June 2018. MMWR Morb Mortal Wkly Rep 2018;67:1189–94. CrossRef PubMed
- Lickness JS, Gardner T, Diop OM, et al. Surveillance to track progress toward polio eradication—worldwide, 2018–2019. MMWR Morb Mortal Wkly Rep 2020;69:623–9. CrossRef PubMed
- Li L, Ivanova O, Driss N, et al. Poliovirus excretion among persons with primary immune deficiency disorders: summary of a seven-country study series. J Infect Dis 2014;210(Suppl 1):S368–72. CrossRef PubMed
- Macklin G, Liao Y, Takane M, et al.; iVDPV Working Group. Prolonged excretion of poliovirus among individuals with primary immunodeficiency disorder: an analysis of the World Health Organization Registry. Front Immunol 2017;8:1103. CrossRef PubMed
- Hampton LM, Farrell M, Ramirez-Gonzalez A, et al.; Immunization systems management group of the Global Polio Eradication Initiative. Cessation of trivalent oral poliovirus vaccine and introduction of inactivated poliovirus vaccine—worldwide, 2016. MMWR Morb Mortal Wkly Rep 2016;65:934–8. CrossRef PubMed
- Al-Mousa H, Al-Saud B. Primary immunodeficiency diseases in highly consanguineous populations from Middle East and North Africa: epidemiology, diagnosis, and care. Front Immunol 2017;8:678. CrossRef PubMed
- Global Polio Eradication Initiative. Guidelines for implementing poliovirus surveillance among patients with primary immunodeficiency disorders (PIDs). Geneva, Switzerland: World Health Organization; 2019.https://www.who.int/immunization/sage/meetings/2019/april/2_Guidelines_Implementing_PID_Suveillance.pdf?ua=1
- Copelyn J, Hincks JR, Wilmshurst JM, et al. Clearance of immunodeficiency-associated vaccine-derived poliovirus infection with Pocapavir. Pediatr Infect Dis J 2020;39:435–7. CrossRef PubMed
Abbreviations: AGG = agammaglobulinemia; HGG = hypogammaglobulinemia; ICF = centromeric region instability, facial anomalies syndrome; MHC = major histocompatibility complex; SCID = severe combined immunodeficiency; XLA = X-linked agammaglobulinemia.
*Data as of May 17, 2020.
 FIGURE. Immunodeficiency-associated vaccine-derived poliovirus (iVDPV) cases reported, by year and VDPV serotype — worldwide, 2010–2019
FIGURE. Immunodeficiency-associated vaccine-derived poliovirus (iVDPV) cases reported, by year and VDPV serotype — worldwide, 2010–2019
Abbreviations: AFP = acute flaccid paralysis; AGG = agammaglobulinemia; CID = combined immunodeficiency disorder; MHC = major histocompatibility complex; OPV = oral poliovirus vaccine; PID = primary immunodeficiency disorder; SCID = severe combined immunodeficiency disorder.
* Data as of May 17, 2020.
† Percentage of divergence is estimated from the number of nucleotide differences in the capsid protein VP1 region from the corresponding parental OPV strain in the latest iVDPV sequence available.
§ Coverage with 3 doses of OPV, based on 2018 data from the World Health Organization (WHO) Vaccine Preventable Diseases Monitoring System (2018 global summary) and WHO-United Nations Children’s Fund coverage estimates https://www.who.int/gho/immunization/poliomyelitis/en/. National data might not reflect weaknesses at subnational levels.
¶ Duration of iVDPV replication was estimated from clinical record by assuming that exposure was from last known receipt of OPV (or date of birth where vaccination data was not available).
** Outcome as of last reported information.
Suggested citation for this article: Macklin G, Diop OM, Humayun A, et al. Update on Immunodeficiency-Associated Vaccine-Derived Polioviruses — Worldwide, July 2018–December 2019. MMWR Morb Mortal Wkly Rep 2020;69:913–917. DOI: http://dx.doi.org/10.15585/mmwr.mm6928a4.
MMWR and Morbidity and Mortality Weekly Report are service marks of the U.S. Department of Health and Human Services.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All HTML versions of MMWR articles are generated from final proofs through an automated process. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (https://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.