Progress Toward Measles Elimination — European Region, 2009–2018
Weekly / May 3, 2019 / 68(17);396–401
Laura A. Zimmerman, MPH1; Mark Muscat, MD, PhD2; Simarjit Singh, MSc2; Myriam Ben Mamou, MD2; Dragan Jankovic, MD2; Siddhartha Datta, MD2; James P. Alexander, MD1; James L. Goodson, MPH1; Patrick O’Connor, MD2 (View author affiliations)
View suggested citationSummary
What is already known about this topic?
Many countries in the World Health Organization European Region (EUR) have made substantial progress toward measles elimination.
What is added by this report?
By end of 2017, 37 (70%) EUR countries had sustained interruption of measles transmission for ≥36 months and were verified to have eliminated endemic measles. During 2017–2018, however, a resurgence of measles occurred in EUR, with large-scale outbreaks in Ukraine, Serbia, and some countries that had achieved elimination.
What are the implications for public health practice?
To achieve regional measles elimination, measures are needed to strengthen immunization programs to achieve high population immunity, maintain high-quality surveillance for rapid case detection, and ensure outbreak preparedness and prompt response to contain outbreaks.
Altmetric:
In 2010, all 53 countries* in the World Health Organization (WHO) European Region (EUR) reconfirmed their commitment to eliminating measles and rubella and congenital rubella syndrome (1); this goal was included as a priority in the European Vaccine Action Plan 2015–2020 (2). The WHO-recommended elimination strategies in EUR include 1) achieving and maintaining ≥95% coverage with 2 doses of measles-containing vaccine (MCV) through routine immunization services; 2) providing measles and rubella vaccination opportunities, including supplementary immunization activities (SIAs), to populations susceptible to measles or rubella; 3) strengthening surveillance by conducting case investigations and confirming suspected cases and outbreaks with laboratory results; and 4) improving the availability and use of evidence for the benefits and risks associated with vaccination (3). This report updates a previous report (4) and describes progress toward measles elimination in EUR during 2009–2018. During 2009–2017, estimated regional coverage with the first MCV dose (MCV1) was 93%–95%, and coverage with the second dose (MCV2) increased from 73% to 90%. In 2017, 30 (57%) countries achieved ≥95% MCV1 coverage, and 15 (28%) achieved ≥95% coverage with both doses. During 2009–2018, >16 million persons were vaccinated during SIAs in 13 (24%) countries. Measles incidence declined to 5.8 per 1 million population in 2016, but increased to 89.5 in 2018, because of large outbreaks in several EUR countries. To achieve measles elimination in EUR, measures are needed to strengthen immunization programs by ensuring ≥95% 2-dose MCV coverage in every district of each country, offering supplemental measles vaccination to susceptible adults, maintaining high-quality surveillance for rapid case detection and confirmation, and ensuring effective outbreak preparedness and response.
Immunization Activities
Since 2002, all 53 countries in EUR have included 2 MCV doses in routine childhood vaccination schedules. WHO and the United Nations Children’s Fund (UNICEF) estimate vaccination coverage for all countries in the region using annual, government-reported administrative coverage data (calculated as the number of doses administered divided by the estimated target population) and vaccination coverage surveys (5). During 2009–2017, annual estimates of MCV1 coverage were available for all 53 countries, and the number of countries with annual MCV2 coverage estimates increased from 47 (89%) to 52 (98%). During 2009–2017, regional coverage estimates for MCV1 and MCV2 ranged from 93% to 95% and 73% to 90%, respectively (Figure). In 2017, 30 (57%) countries achieved ≥95% MCV1 coverage, and 15 (28%) had ≥95% estimated coverage with both doses (Table 1). During 2009–2017, >16 million persons were vaccinated in 21 SIAs conducted in 13 countries (Supplementary Table, https://stacks.cdc.gov/view/cdc/77666). Reported administrative vaccination coverage was ≥95% in nine (43%) SIAs, and the weighted average SIA coverage was 88%; no post-SIA coverage surveys were reported.
Surveillance Activities
Measles surveillance data are reported monthly to WHO from all EUR countries either directly or via the European Centre for Disease Prevention and Control.† As of 2018, 47 (89%) countries report case-based measles surveillance data; six (11%)§ report aggregate data. Suspected measles cases are investigated and classified as laboratory-confirmed, epidemiologically linked (to a laboratory-confirmed case), clinically compatible, or discarded (a suspected case that does not meet the clinical or laboratory definition) (6). The WHO European Measles and Rubella Laboratory Network provides laboratory confirmation and genotyping of measles virus isolates from patients with reported cases (7). Key measles case-based surveillance performance indicators include 1) the number of suspected cases discarded as nonmeasles or nonrubella (target: ≥2 per 100,000 population); 2) the percentage of case investigations conducted within 48 hours of report (target: ≥80%); 3) the percentage of suspected cases (excluding those that are epidemiologically linked) with an adequate specimen collected within 28 days of rash onset and tested in a WHO-accredited or proficient laboratory (target: ≥80%); and 4) the percentage of cases for which the origin of infection (i.e., the source of the virus) is determined (target: ≥80%). During 2009–2018, the number of EUR countries that met the target for suspected cases discarded as nonmeasles at the national level increased from one (3%) in 2009 to 10 (21%) in 2018 (Table 2). From 2009 to 2018, the number of countries achieving the targets for timely investigations of suspected cases and adequate specimen collection increased from one (3%) to 24 (51%) and from 13 (36%) to 38 (81%), respectively.
Measles Incidence and Genotypes
During 2009–2018, annual regional measles incidence varied from 8.8 per 1 million population (7,884 cases) in 2009 to an average of 30.1 (average 28,021 cases) during 2010–2015. Incidence declined to a low of 5.8 (5,273 cases) in 2016, before increasing approximately fourteenfold to a high of 89.5 (82,596 cases) in 2018 (Table 1) (Figure). These 82,596 cases were reported from 47 (89%) EUR countries; 73,295 (89%) were reported by eight countries: Ukraine (53,218 cases; 64% of total); Serbia (5,076; 6%); France (2,913; 4%); Israel (2,919; 4%); Georgia (2,203; 3%); Greece (2,193; 3%); Italy (2,517; 3%); and Russia (2,256; 3%). The highest measles incidences in 2018 were in Ukraine (1,209.2 per 1 million) and Serbia (579.3). Among all measles cases reported in 2018, adults aged ≥20 years accounted for 30,561 (37%). The countries with the highest proportions of adult measles cases were Italy (68%), Serbia (67%), and Russia (42%). Among 179 measles deaths reported in EUR countries during 2009–2018, 114 (64%) occurred during 2017–2018, including 93 (82%) from four countries: Romania (46), Ukraine (20), Serbia (15), and Italy (12). EUR reported 17,587 measles virus sequences to the WHO global measles nucleotide surveillance database. The most predominant measles virus genotypes detected were D4 (21% overall, 66% during 2009–2012), D8 (45% overall, 76% during 2013–2016), and B3 (33% overall, 58% during 2017–2018) (8) (Supplementary Figure, https://stacks.cdc.gov/view/cdc/77667).
Regional Verification of Measles Elimination
The European Regional Verification Commission for Measles and Rubella Elimination was established in 2011 to evaluate the status of measles and rubella elimination¶ in EUR countries based on documentation submitted annually by national verification committees (1). By the end of 2017, 43 (91%) countries had interrupted endemic measles virus transmission for ≥12 months, including 37 (70%)** that had sustained interruption for ≥36 months and were verified to have eliminated endemic measles virus transmission (8).
Discussion
After relatively stable albeit high measles incidence in EUR during 2009–2016, the number of reported measles cases tripled from 2017 to 2018, including outbreaks in eight countries reporting >2,000 measles cases each. The 2018 measles resurgence was attributable to measles virus transmission that began in 2017 and continued during 2018 in France, Greece, Romania, Russia, Serbia, and Ukraine. In addition, measles virus importations followed by widespread measles virus transmission occurred in countries that had achieved elimination, including Albania, Belarus, Czech Republic, Israel, and Montenegro. Despite high reported national coverage, factors associated with the resurgence included persistent measles virus reservoirs in EUR countries with limited resources and weak immunization systems, an accumulation of susceptible young children in marginalized communities with suboptimal coverage, and an accumulation of susceptible young adults who had escaped both natural measles infection and measles vaccination over a prolonged period of decreased measles incidence.
Outbreak response differed among countries. In some countries, large outbreaks caused substantial financial and human resource burdens, which resulted in delayed or inadequate outbreak responses and ongoing disease transmission. In other countries, outbreak response vaccination campaigns were not implemented because of insufficient political commitment, poor acceptance of mass immunization by health authorities and the public, lack of infrastructure to vaccinate specific susceptible population groups, and vaccine supply challenges. To achieve better outbreak control, countries in the region will need to adhere to their commitment to eliminate measles and rubella and ensure that dedicated financial and human resources are available for strong vaccination and surveillance programs, including outbreak preparedness and response.
The measles resurgence and the European Vaccine Action Plan midterm review in 2018 (9) highlighted ongoing challenges, including inadequate vaccine delivery infrastructure in some middle-income countries that resulted in suboptimal vaccination coverage and vaccine stock-outs; prevalent antivaccine sentiment; large populations of unvaccinated persons, including ethnic and religious minorities and adults; an increased proportion of cases in persons aged ≥20 years, who are difficult to reach with routine immunization services; and nosocomial outbreaks that affected patients and health care personnel with spread to the community.
To address these challenges and accelerate measles elimination efforts in EUR, the European Regional Office has targeted the following areas for action: 1) achieving and maintaining ≥95% vaccination coverage; 2) improving understanding of barriers to vaccination in vulnerable groups and increasing vaccine demand; 3) closing immunity gaps in the population through innovative and locally tailored approaches; 4) ensuring high-quality measles surveillance for rapid case detection and targeted outbreak response activities; and 5) strengthening infection prevention and control practices, particularly during outbreaks. The midterm review also highlighted the recent recommendation by the WHO Strategic Advisory Group of Experts on Immunization that countries institutionalize school entry checks to close immunity gaps as a key strategy for achieving measles elimination (10).
The findings in this report are subject to at least two limitations. First, surveillance data likely underestimate actual disease incidence because not all patients seek care, and it is likely that not all cases are reported. Second, measles surveillance performance and data quality vary among countries in the region, which might have led to reporting bias for some countries.
In EUR, 70% of countries have been verified as having achieved measles elimination; however, the recent resurgence highlighted challenges to achieving and maintaining elimination. All countries need to strengthen immunization programs to achieve and sustain high population immunity, maintain high-quality surveillance, and ensure outbreak preparedness and prompt response to contain outbreaks. Elimination efforts that focus on reaching vulnerable communities and adults will likely provide opportunities to improve access to vaccination services for all and help achieve European Vaccine Action Plan and future universal health goals.
Corresponding author: Laura A. Zimmerman, LZimmerman@cdc.gov, 404-639-8690.
1Global Immunization Division, Center for Global Health, CDC; 2Vaccine Preventable Diseases and Immunization, European Regional Office, World Health Organization, Copenhagen, Denmark.
All authors have completed and submitted the ICMJE form for disclosure of potential conflicts of interest. No potential conflicts of interest were disclosed.
* The European Region, with a population of approximately 900 million, is one of six WHO regions and consists of 53 countries: Albania, Andorra, Armenia, Austria, Azerbaijan, Belarus, Belgium, Bosnia and Herzegovina, Bulgaria, Croatia, Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Georgia, Germany, Greece, Hungary, Iceland, Ireland, Israel, Italy, Kazakhstan, Kyrgyzstan, Latvia, Lithuania, Luxembourg, Malta, Monaco, Montenegro, Netherlands, North Macedonia, Norway, Poland, Portugal, Republic of Moldova, Romania, Russia, San Marino, Serbia, Slovakia, Slovenia, Spain, Sweden, Switzerland, Tajikistan, Turkey, Turkmenistan, Ukraine, United Kingdom, and Uzbekistan.
† For Iceland, Norway, and the 28 member states of the European Union (Austria, Belgium, Bulgaria, Croatia, Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Latvia, Lithuania, Luxembourg, Malta, Netherlands, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, Sweden, and the United Kingdom).
§ Belgium, Bosnia and Herzegovina, Kazakhstan, North Macedonia, Serbia, and Ukraine report aggregated surveillance data to WHO.
¶ Elimination defined as interruption of endemic measles transmission for >36 months in the presence of a well-functioning surveillance system.
** Countries that had interrupted endemic measles virus transmission for >12 months include Albania, Andorra, Armenia, Azerbaijan, Belarus, Bulgaria, Croatia, Cyprus, Czech Republic, Denmark, Greece, Estonia, Finland, Hungary, Iceland, Ireland, Israel, Latvia, Lithuania, Luxembourg, Malta, Montenegro, Netherlands, North Macedonia, Norway, Monaco, Portugal, Republic of Moldova, San Marino, Slovakia, Slovenia, Spain, Sweden, Tajikistan, Turkmenistan, United Kingdom, and Uzbekistan.
References
- World Health Organization Regional Health Office for Europe. Regional Committee for Europe Sixtieth Session, September 16, 2010. Copenhagen, Denmark: World Health Organization Regional Office for Europe; 2010. http://www.euro.who.int/__data/assets/pdf_file/0016/122236/RC60_eRes12.pdf?ua=1
- World Health Organization Regional Office for Europe. European vaccine action plan 2015–2020. Copenhagen, Denmark: World Health Organization Regional Office for Europe; 2014. http://www.euro.who.int/en/health-topics/disease-prevention/vaccines-and-immunization/publications/2014/european-vaccine-action-plan-20152020-2014
- World Health Organization Regional Office for Europe. Eliminating measles and rubella: framework for the verification process in the WHO European Region. Copenhagen, Denmark: World Health Organization Regional Office for Europe; 2014. http://www.euro.who.int/__data/assets/pdf_file/0009/247356/Eliminating-measles-and-rubella-Framework-for-the-verification-process-in-the-WHO-European-Region.pdf?ua=1
- CDC. Progress toward measles elimination—European Region, 2005–2008. MMWR Morb Mortal Wkly Rep 2009;58:142–5. PubMed
- World Health Organization. Immunization, vaccines and biologicals: data, statistics and graphics. Geneva, Switzerland: World Health Organization; 2018. https://www.who.int/immunization/monitoring_surveillance/data/en/
- World Health Organization Regional Office for Europe. Surveillance guidelines for measles, rubella and congenital rubella syndrome in the WHO European Region. Copenhagen, Denmark: World Health Organization Regional Office for Europe; 2012. http://www.euro.who.int/__data/assets/pdf_file/0018/79020/e93035-2013.pdf
- World Health Organization. Meeting report. 13th Meeting of the Measles/Rubella Regional Reference Laboratories. March 15–16, 2018, Copenhagen, Denmark. http://www.euro.who.int/__data/assets/pdf_file/0008/387161/rrl-mar-2018-meeting-eng.pdf
- World Health Organization. Genetic diversity of wild-type measles viruses and the global measles nucleotide surveillance database (MeaNS). Wkly Epidemiol Rec 2015;90:373–80. PubMed
- World Health Organization Regional office for Europe. Report of the 7th Meeting of the European Regional Verification Commission for Measles and Rubella Elimination (RVC), June 13–15, 2018, Paris, France. http://www.euro.who.int/en/health-topics/communicable-diseases/measles-and-rubella/publications/2018/7th-meeting-of-the-european-regional-verification-commission-for-measles-and-rubella-elimination-rvc.-report
- World Health Organization Regional Office for Europe. European vaccine action plan midterm report. Copenhagen, Denmark: World Health Organization Regional Office for Europe; 2018. http://www.euro.who.int/en/health-topics/disease-prevention/vaccines-and-immunization/publications/2018/european-vaccine-action-plan-midterm-report
 FIGURE. Estimated coverage with the first and second doses of measles-containing vaccine* and the number of confirmed measles cases† — World Health Organization (WHO) European Region, 2009–2018§
FIGURE. Estimated coverage with the first and second doses of measles-containing vaccine* and the number of confirmed measles cases† — World Health Organization (WHO) European Region, 2009–2018§
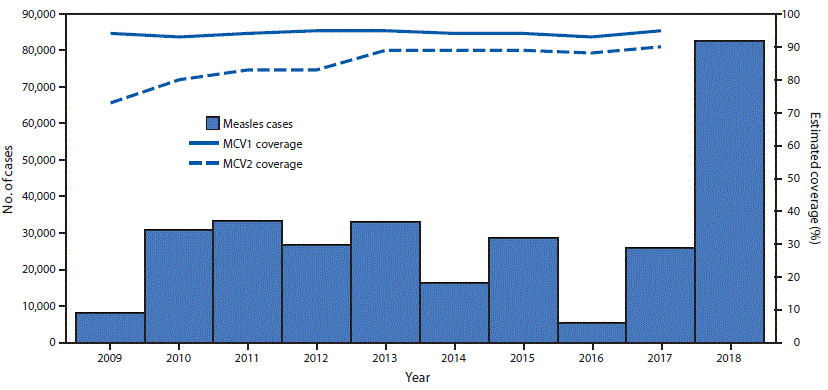
Abbreviations: MCV1 = first dose of a measles-containing vaccine; MCV2 = second dose of a measles-containing vaccine.
* WHO and United Nations Children’s Fund estimates, July 15, 2018, update. https://www.who.int/immunization/monitoring_surveillance/data/en/.
† Cases reported to WHO, as of March 1, 2019. https://www.who.int/immunization/monitoring_surveillance/data/en/.
§ Date range for estimated coverage = 2009–2017; date range for confirmed measles cases = 2009–2018.
Abbreviations: MCV1 = first dose of MCV; MCV2 = second dose of MCV; NR = not reported (country did not report coverage for the year specified).
* WHO and United Nations Children’s Fund estimates of national immunization coverage, 2018. https://www.who.int/immunization/monitoring_surveillance/data/en/.
† Includes confirmed cases by laboratory or epidemiologic linkage and clinically compatible cases meeting the WHO clinical case definition of measles for which no adequate specimen was collected and that cannot be epidemiologically linked to a laboratory-confirmed case of measles.
§ MCV schedule is the 2017 schedule.
¶ Per 1 million population.
** 2018 MCV1 and MCV2 coverage estimates not available.
†† Also recommended for males aged 16–17 years who have not previously received 2 MCV doses.
§§ Catch-up vaccination at age 15 years is also performed.
¶¶ Catch-up monovalent measles vaccine is also recommended for persons aged 18–55 years.
* Percentage of measles or rubella routine surveillance reports submitted from subnational to national level.
† Percentage of measles or rubella routine surveillance reports submitted from subnational to national level by the deadline set by national program.
§ The rate of suspected measles or rubella cases investigated and discarded as nonmeasles and nonrubella, using laboratory testing in a proficient laboratory or epidemiological linkage to another confirmed disease.
¶ Percentage of suspected measles or rubella cases with an adequate case investigation initiated within 48 hours of case notification.
** Percentage of suspected measles or rubella cases with an adequate specimen collected and tested in a WHO-accredited or proficient laboratory.
†† Percentage of confirmed measles or rubella cases for which the origin of infection (i.e., source of virus) has been identified.
Suggested citation for this article: Zimmerman LA, Muscat M, Singh S, et al. Progress Toward Measles Elimination — European Region, 2009–2018. MMWR Morb Mortal Wkly Rep 2019;68:396–401. DOI: http://dx.doi.org/10.15585/mmwr.mm6817a4.
MMWR and Morbidity and Mortality Weekly Report are service marks of the U.S. Department of Health and Human Services.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All HTML versions of MMWR articles are generated from final proofs through an automated process. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (https://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.