Key points
- Measles is one of the most contagious diseases. MMR vaccine provides the best protection.
- Isolate infected patients for 4 days after they develop a rash and follow airborne precautions in healthcare settings.
- Report suspected measles cases to your local health department.
- Laboratory confirmation is essential for all sporadic measles cases and all outbreaks.
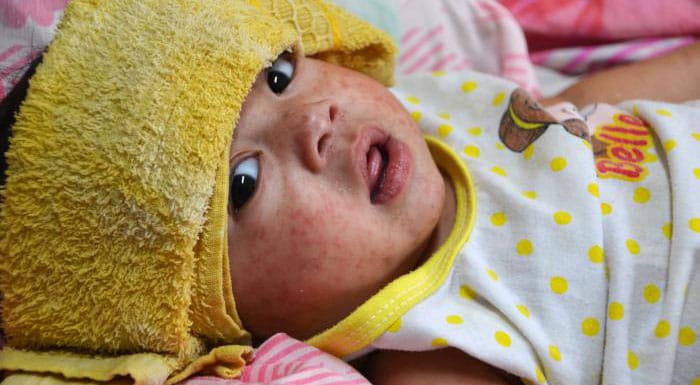
Clinical features
Measles is an acute viral respiratory illness. It is characterized by:
- A prodrome of fever (as high as 105°F), malaise, and cough, coryza, and conjunctivitis (three "C"s)
- A pathognomonic enanthema (Koplik spots)
- Followed by a maculopapular rash
The rash usually appears about 14 days after a person is exposed. The rash spreads from the head to the trunk to the lower extremities.
Patients are considered to be contagious from 4 days before to 4 days after the rash appears. Sometimes immunocompromised patients do not develop the rash.
Earn continuing education (CE)
To receive continuing education (CE) for this course, Clinical Overview of Measles: Diagnosis, Laboratory Testing, and Outbreak Response (Web on Demand) - WD4728, please visit CDC TRAIN and search for the course in the Course Catalog. Then, follow the steps below.
- Register for and complete the course.
- Pass the post-assessment with a percentage of 80%.
- Complete the evaluation.
- Visit Your Learning to access your certificates and transcript.
Complications
Common complications from measles include otitis media, bronchopneumonia, laryngotracheobronchitis (croup), and diarrhea. Measles infection can also be complicated by secondary bacterial infections, which can cause tonsillitis, otitis media, and pneumonia.
Even in previously healthy children, measles can cause serious illness requiring hospitalization.
- 1 out of every 1,000 measles cases will develop acute encephalitis, which often results in permanent brain damage.
- 1 to 3 out of every 1,000 children who become infected with measles will die from respiratory and neurologic complications.
- Subacute sclerosing panencephalitis (SSPE) is a rare, but fatal degenerative disease of the central nervous system characterized by:
- Behavioral and intellectual deterioration.
- Seizures that generally develop 7 to 10 years after measles infection.
Who is at risk for complications
People at high risk for complications include:
- Infants and children aged <5 years
- Adults aged >20 years
- Pregnant women
- People with weakened immune systems, such as from leukemia and HIV infection
Pregnant women and congenital measles
Measles during pregnancy can result in adverse outcomes for pregnant women, such as pneumonia and death. Measles can also lead to adverse pregnancy outcomes, including pregnancy loss, premature delivery, and low birth weight.
Measles can also be transmitted to a fetus during pregnancy. Measles exposure in utero can result in congenital measles infection of the newborn, which is characterized by a febrile rash syndrome within 10 days of birth. Congenital measles can result in severe complications, including encephalitis and death, and is associated with a higher risk of SSPE. Providing immune globulin to the neonate may prevent or attenuate congenital measles and reduce the risk of neonatal mortality due to congenital measles.
Additional resources:
Cause
Measles is caused by a single-stranded, enveloped RNA virus with 1 serotype. It is classified as a member of the genus Morbillivirus in the Paramyxoviridae family. Humans are the only natural hosts of measles virus.
How it spreads
Measles is one of the most contagious of all infectious diseases. Up to 9 out of 10 susceptible people with close contact to a measles patient will develop measles.
The virus is transmitted by:
- Direct contact with infectious droplets.
- Airborne spread when an infected person breathes, coughs, or sneezes.
Measles virus can remain infectious in the air for up to 2 hours after an infected person leaves an area.
Disease rates
Prior to vaccine introduction:
The live measles vaccine was licensed in 1963. In the decade before, an average of 549,000 measles cases and 495 measles deaths were reported annually in the United States. However, it is likely that 3 to 4 million people on average were infected with measles annually; most cases were not reported. Of the reported cases, approximately:
- 48,000 people were hospitalized from measles.
- 1,000 people developed chronic disability from acute encephalitis caused by measles annually.
Post-vaccine era:
In 2000, measles was declared eliminated from the United States. However, cases and outbreaks still occur every year in the United States. This is because measles is still commonly transmitted in other countries in Europe, the Middle East, Asia, the Americas, and Africa.
Since 2000, the annual number of cases has ranged from a low of 37 in 2004; to a high of 1,282 in 2019. The majority of cases in the United States have been among people who are not vaccinated against measles.
Prevention
Measles can be prevented with measles-containing vaccine. Measles vaccine is usually administered as the combination measles, mumps, and rubella (MMR) vaccine. The combination measles, mumps, rubella, and varicella (MMRV) vaccine can be used for children aged 12 months through 12 years.
CDC recommends children get 2 doses of MMR vaccine. Teens and adults should also be up to date on their MMR vaccination. Almost everyone who does not respond to the measles component of the first dose of MMR vaccine at age 12 months or older will respond to the second dose. Therefore, the second dose of MMR is administered to address primary vaccine failure.
While the most important measure to prevent measles transmission in all settings is ensuring community immunization, core measles prevention in healthcare settings requires a multi-faceted approach.
Diagnosis and laboratory testing
Consider measles in patients presenting with febrile rash illness and clinically compatible measles symptoms, especially if they recently traveled internationally; or were exposed to a person with febrile rash illness. Healthcare providers are required to report suspected measles cases to their local health department.
Laboratory confirmation is essential for all sporadic measles cases and all outbreaks. The most common methods for confirming measles infection are:
- Detection of measles-specific IgM antibody in serum.
- Measles RNA by RT-PCR in a respiratory specimen.
Obtain both a serum sample and a throat swab (or nasopharyngeal swab) from patients suspected to have measles at first contact with them. Urine samples may also contain virus. When feasible to do so, collecting both respiratory and urine samples can increase the likelihood of detecting measles virus.
Molecular analysis can also be conducted to determine the genotype of the measles virus. Genotyping is used to map the transmission pathways of measles viruses. The genetic data can help to link or unlink cases and can suggest a source for imported cases. Genotyping is the only way to distinguish between wild-type measles virus infection and a rash caused by a recent measles vaccination.
Patient management
There is no specific antiviral therapy that is Food and Drug Administration (FDA)-approved for treatment of measles. Medical care is generally supportive and helps to relieve symptoms. Complications such as pneumonia and secondary bacterial infections should be promptly managed under the supervision of a healthcare provider.
Vitamin A does not prevent measles and is not a substitute for vaccination. Consistent with guidance from the American Academy of Pediatrics, vitamin A may be administered to infants and children in the United States with measles under the supervision of a healthcare provider as part of supportive management. Children with severe measles, such as those who are hospitalized, should be managed with vitamin A. Overuse of Vitamin A can lead to toxicity and cause damage to the liver, bones, central nervous system, and skin. Pregnant women should avoid taking high levels of vitamin A as it has been linked to severe birth defects.
If vitamin A is recommended, it should be administered immediately upon diagnosis and repeated the next day for a total of 2 doses under the supervision of a healthcare provider. The recommended age-specific daily doses are:
- 50,000 IU for infants younger than 6 months of age
- 100,000 IU for infants 6–11 months of age
- 200,000 IU for children 12 months of age and older
People who are infected should be isolated for 4 days after they develop a rash. Airborne precautions should be followed in healthcare settings.
The preferred placement for patients who require airborne precautions is in a single-patient airborne infection isolation room (AIIR). Regardless of presumptive immunity status, all healthcare staff entering the room should use respiratory protection consistent with airborne infection control precautions. This includes use of an N95 respirator or a respirator with similar effectiveness in preventing airborne transmission.
There is no evidence to support routine use of antibiotics for measles treatment. Measles may be complicated by secondary bacterial infections for which antibiotic treatment is indicated. Treatment decisions for infections should be based on the clinical assessment of a healthcare provider, taking into account type of infection, illness severity, and other patient factors.
Ribavirin is a broad-spectrum antiviral that is approved by the U.S. Food and Drug Administration (FDA) for management of chronic hepatitis C infection and for the treatment of hospitalized infants and young children with severe lower respiratory tract infections due to respiratory syncytial virus. Ribavirin demonstrates in vitro activity against measles virus and has been used to treat patients with measles who are severely immunocompromised or patients with severe measles disease such as pneumonia and encephalitis. However, clinical data are lacking regarding its efficacy.
Ribavirin is not approved by the FDA to treat measles. Oral ribavirin and an inhalation solution for aerosolization are available commercially and intravenous ribavirin is available only through an Emergency Investigational New Drug (EIND) application via FDA. Case reports and small case series have generally reported on use of oral or intravenous ribavirin for measles treatment, with few reports available regarding use of inhaled ribavirin.
Measles importation and outbreaks
Measles cases occur as a result of importations by people who were infected while in other countries; and from subsequent transmission that may occur from those importations. Measles is more likely to spread and cause outbreaks in communities where groups of people are unvaccinated.
Outbreaks in countries to which Americans often travel can directly contribute to an increase in measles cases in the United States. In recent years, measles importations have come from frequently visited countries and countries where large outbreaks were reported. These include but not limited to the Philippines, Ukraine, Israel, Thailand, Vietnam, England, France, Germany, and India.
Resources and tools
Webinars & trainings
- Be on Alert for Travel-Related Measles | AMA Ed Hub
- Clinical Overview of Measles: Diagnosis, Laboratory Testing, and Outbreak Response (Web on Demand)
- You Call the Shots: Measles, Mumps, and Rubella
- Pink Book Web-on-Demand: Measles, Mumps, and Rubella Vaccines