NPCR Timeline
1990
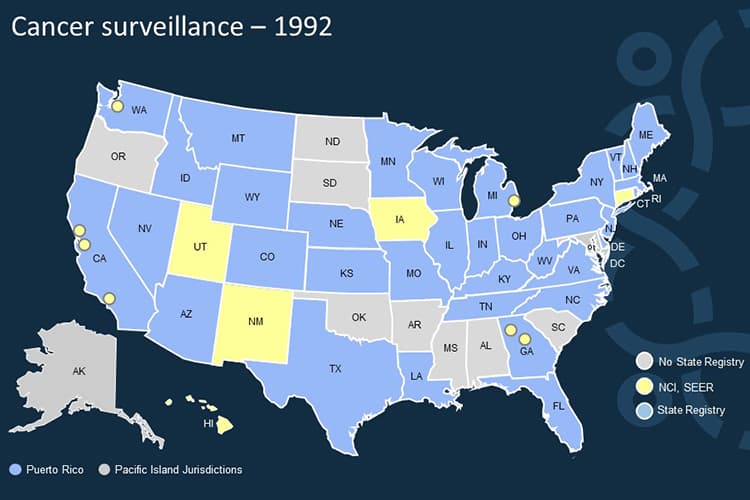
Before CDC’s National Program of Cancer Registries (NPCR) was established, 10 states had no registry, and most states with registries lacked the resources and legislative support they needed to gather complete data. A centralized national program to collect cancer data is needed.
1992
The Cancer Registries Amendment Act is passed to establish CDC support for statewide registries to collect basic data on cancer, such as incidence, stage, and treatment. This act created the CDC’s National Program for Central Cancer Registries (NPCR).
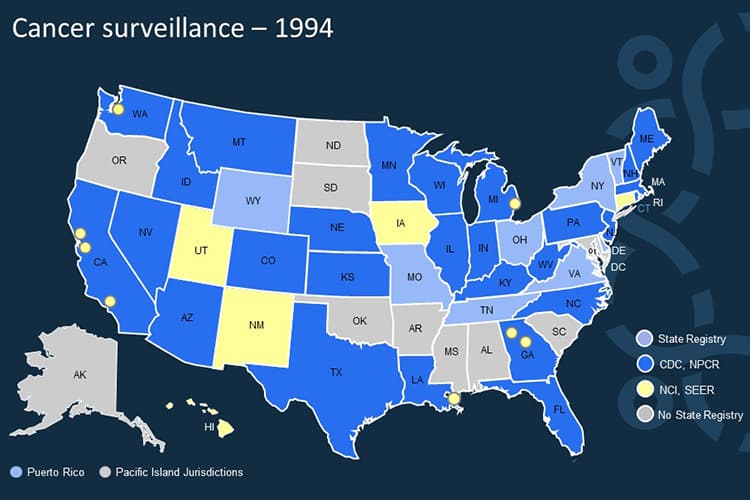
1994
By the end of 1994, 26 states have received funding through NPCR.
1994
To facilitate data transmission and improve data quality, NPCR develops EDITS, software tools that standardize methods for identifying errors and inconsistencies in cancer registry data.

1996
Abstract Plus is released. This software summarizes medical records into an electronic report of cancer diagnosis and treatment.
1999
NPCR collaborates with the North American Association of Central Cancer Registries (NAACCR), central cancer registries, and a national laboratory to develop a national standard for reporting cancer pathology data to central cancer registries.
1999
Prep Plus is released. This program incorporates NAACCR-formatted abstracts into a central cancer registry’s database.

2000
Central Registry System (CRS) Plus is released. It links, consolidates, and maintains source records in a central cancer registry’s database.
Together with Prep Plus, CRS Plus allows registries to create de-identified records to submit to CDC each year. The software is provided free of charge to NPCR registries.
2000
The NPCR-Cancer Surveillance System (NPCR-CSS) is established to receive, evaluate, and disseminate data from central cancer registries.
2001
In January 2001, NPCR registries begin reporting their cancer incidence data to CDC every year.
2001
Seven NPCR registries complete activities for the Patterns of Care: Breast, Colorectal, & Prostate Cancer Study and participate in the international CONCORD Study. Three NPCR registries receive funding to conduct the Ovarian Cancer Treatment Patterns & Outcomes Study.
2001
All 50 states, the District of Columbia, U.S. Virgin Islands and Puerto Rico have a central cancer registry.
2002
In the fall of 2002, NPCR, the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program, and NAACCR jointly produce the first official federal cancer incidence statistics. The resulting report, U.S. Cancer Statistics: 1999 Incidence [PDF-1.7MB], provides state-specific and regional data for cancer cases diagnosed in 1999. Cancer statistics from 37 states, 6 metropolitan areas, and the District of Columbia are included; these areas contain about 78% of the U.S. population.
2002
Congress authorizes the Benign Brain Tumor Cancer Registries Amendment Act. It requires cancer registries to collect data about non-cancerous brain tumors.

2003
The Indian Health Service (IHS) and CDC start a data linkage project to help registries describe the burden of cancer among American Indian and Alaska Native people more accurately. Data from 25 NPCR registries are linked with data from IHS patient administrative records to improve the classification of American Indian/Alaska Native race in the registries.
2004
NPCR starts the Modeling Electronic Reporting Project (MERP) in collaboration with the Virginia Commonwealth University Health System, the Virginia Cancer Registry, and NCI’s SEER Program. MERP finds the best ways to use the electronic medical record for cancer surveillance reporting.
2005
NPCR establishes the Advancing E-cancer Reporting and Registry Operations (AERRO) project. The Electronic Pathology (ePath) project is part of AERRO. The ePath project helps registries implement electronic reporting of pathology reports and cancer biomarkers using NAACCR guidelines.
2005
Electronic Mapping, Reporting, and Coding (eMaRC) Plus is developed to receive and process Health Level Seven (HL7) files from anatomic pathology laboratories.
2006
NPCR and CDC’s National Center for Health Statistics agree to add data from the National Death Index [PDF-253KB] to USCS.
2007
The Pacific Island Jurisdiction has a central cancer registry.

2008
Congress authorizes the Carolyn Pryce Walker Conquer Childhood Cancer Act. It establishes a national childhood cancer registry and provides support for electronic early case capture of childhood cancer cases.
2009
The American Recovery and Reinvestment Act is passed, increasing support for meaningful use of electronic health records. NPCR is instrumental in establishing electronic reporting to cancer registries as a public health objective.

2010
NPCR begins collecting success stories from funded registries to describe their progress and value.
2016
NPCR and the Food and Drug Administration begin working on a natural language processing (NLP) workbench. It provides access to NLP and machine learning tools needed to develop and share language models that map unstructured clinical text to standardized coded data.
2017
The USCS Data Visualizations tool is released. It displays the official federal statistics on cancer incidence and deaths.
2017
The first USCS Public Use Database is released for cases diagnosed between 2001 and 2014.

2018
Congress passes the Childhood Cancer Survivorship, Treatment, Access and Research (STAR) Act. It reauthorizes the Carolyn Pryce Walker Conquer Childhood Cancer Act and allows CDC to expand ways that laboratories can rapidly report cases of cancer among children, adolescents, and young adults into NPCR registries.
2018
NPCR launches the National Oncology rapid Ascertainment Hub (NOAH). NOAH supports analysis of laboratory reports and processes all data.

2021
NPCR starts work on a data modernization initiative to develop a cloud-based computing platform specifically for cancer data. Laboratories and health care providers successfully sent and (some) central cancer registries received data using the new platform.

2022
NPCR celebrates 30 years of helping central cancer registries collect high-quality data to measure progress, drive action, prevent cancers, and improve treatment for all people.