ACIP Evidence to Recommendations (EtR) for Use of Novavax COVID-19 Vaccine Booster Dose for adults ages 18 years and older under an Emergency Use Authorization
Question: Should a Novavax COVID-19 vaccine booster dose (1 dose, 5µg antigen + 50µg Matrix-M adjuvant, IM, ≥2 months after completion of a COVID-19 vaccine primary series) be recommended for persons ages 18 years and older under an anticipated Emergency Use Authorization?
Population: People 18 years of age and older
Intervention: Novavax COVID-19 vaccine booster dose (1 dose, 5µg antigen + 50µg Matrix-M adjuvant, IM)
Background:
The emergence of SARS-CoV-2, the virus that causes coronavirus disease 2019 (COVID-19), in late 2019 has led to a global pandemic with dramatic societal and economic impact on individual persons and communities. In the United States, more than 96 million cases and more than 1 million COVID-19-associated deaths have been reported as of September 27, 2022.1 Persons of all ages are at risk for infection and severe disease. However, the risk for severe illness from COVID-19 is higher in people aged ≥65 years, those living in long-term care facilities, and those with chronic medical conditions. Additionally, there is a disproportionate burden of COVID-19 infections and deaths among racial and ethnic minority communities. Non-Hispanic Black, Hispanic/Latino (Hispanic) and American Indian/Alaska Native persons have experienced higher rates of disease, hospitalization and death compared with non-Hispanic White persons. This is likely related to inequities in social determinants of health that put racial and ethnic minority groups at increased risk for COVID-19, including overrepresentation among essential workers who have higher risk of exposure to COVID-19, lower incomes, reduced access to healthcare, or higher rates of comorbid conditions.
COVID-19 remains a significant threat, killing nearly 350 Americans per day.2 Being up to date with COVID-19 vaccines continues to provide strong protection against severe disease, hospitalization, and death in adults, including during Omicron variant predominance. However, protection is highest in adults who receive all recommended booster doses.3 About 48% of adults, which is approximately 96 million, are eligible for a booster and have not yet received a booster.4
On July 13, 2022, the Food and Drug Administration (FDA) issued an Emergency Use Authorization (EUA) for the Novavax monovalent COVID-19 vaccine for the prevention of COVID-19 in persons ages 18 years and older. Furthermore, on October 19, 2022, the FDA amended the Emergency Use Authorization (EUA) to allow for use of the Novavax monovalent COVID-19 vaccine as a first booster at least 6 months after completion of a primary series among persons ages 18 years who are unable or unwilling to receive a bivalent booster. Following FDA’s regulatory action, CDC signed a decision memo allowing Novavax monovalent COVID-19 boosters for persons ages 18 years and older on October 19, 2022. This action gives people ages 18 years and older the option to receive a Novavax monovalent booster instead of an updated (bivalent) Pfizer-BioNTech or Moderna booster if they have completed primary series vaccination but have not previously received a COVID-19 booster—and if they cannot or will not receive mRNA vaccines.
Additional background information supporting the interim ACIP recommendation on the use of Novavax COVID-19 vaccine can be found in the relevant publication of the recommendation referenced on the ACIP website.
__________________________
1 https://covid.cdc.gov/covid-data-tracker/#trends_dailycases
2 https://covid.cdc.gov/covid-data-tracker/#trends_dailydeaths_select_00 Accessed October 13, 2022.
3 https://covid.cdc.gov/covid-data-tracker/#vaccine-effectiveness Accessed October 13, 2022.
4 https://covid.cdc.gov/covid-data-tracker/#vaccinations_vacc-people-additional-dose-totalpop Data as of October 6, 2022, at 06:00am ET. Accessed October 13, 2022.
Updates to the EtR framework are presented below to address the evidence supporting the Novavax booster in persons ages 18 years and older. Please Note: There was not an ACIP vote for this EtR. Therefore, while the Work Group reviewed data to support the recommendation, they did not determine their judgement for any of the domains listed below:
Benefits and Harms
| Criteria | Evidence | Additional Information |
|---|---|---|
| How substantial are the desirable anticipated effects? | In an open-label continuation of the phase 3 randomized controlled trial among persons ages 18 years and older in the United States and Mexico, 12,777 participants received booster doses as of the data cut off (March 26, 2022). No clinical trial efficacy or real-world vaccine effectiveness data is yet available reflective of Novavax monovalent COVID-19 vaccine effectiveness against Omicron, or against Omicron BA.4/5. However, 298 participants provided immunogenicity data and the data suggest that receipt of a Novavax booster dose induces enhanced immune response to both ancestral strain (geometric mean fold rise [GMFR]: 3.4 [95% CI: 2.8, 4.0) and to Omicron BA.1 (geometric mean fold rise [GMFR]: 17.3 [95% CI: 10.6, 28.1) when compared with the Novavax primary series.1 | |
| How substantial are the undesirable anticipated effects? | Data to date do not demonstrate concerning safety signals. Rare occurrences of myocarditis or pericarditis were identified in primary series clinical trials and in global post marketing data preceding primary series authorization in the United States. A single case of myocarditis was evident post-booster in clinical trials to date. Additional cases of myocarditis and pericarditis have occurred after Novavax COVID-19 vaccine in Australia, but cases after Novavax COVID-19 vaccine remain rare globally.
Furthermore, reactogenicity data following dose 2 and booster dose (dose 3) were available in 124 participants. Local and systemic reactogenicity appeared to be slightly more common after the booster dose than after dose 2, with severe (grade ≥3) systemic reactions reported in 23.2% and 15.1% of participants, respectively. Serious adverse events after the booster dose were reported by 43/12,777 participants (0.3%). No deaths occurred related to the booster dose.1
|
Values and Acceptability
| Criteria | Evidence | Additional Information |
|---|---|---|
| Does the target population feel that the desirable effects are large relative to undesirable effects? | In a survey designed by the CDC and University of Iowa/RAND Corporation to assess vaccination intentions for a protein-based COVID vaccine with or without adjuvant among unvaccinated Americans, 16% of unvaccinated respondents reported that they “probably” or “definitely” would get an adjuvanted protein-based COVID-19 vaccine. Additionally, vaccination intentions were significantly higher among men (21.9%) than among women (11.9%). However, vaccine intentions did not vary by US region, metropolitan status, age, or education.1 | The survey collected data from January 27 – February 2, 2022, with a sample size of 541 respondents.1 |
| Is there important uncertainty about or variability in how much people value the main outcomes? | In relation to vaccination intentions for an adjuvanted protein subunit COVID vaccine among unvaccinated adults in the United States from January – February 2022, vaccination intentions were significantly lower among non-Hispanic White adults (9.6%) than among non-Hispanic Black adults (20.1%) or among Hispanic adults (19.5%).1 |
Feasibility and Resource Use
| Criteria | Evidence | Additional Information |
|---|---|---|
| Is the intervention feasible to implement? | Despite delivery of 841,300 doses, Novavax uptake has been limited in the United States. As of October 6, 2022, 32,992 doses have been administered since primary series authorization in July 2022.1
Additionally, Novavax booster doses are of exactly the same formulation and dose as primary series doses, which means there is no “learning curve” for providers. Doses have been purchased by the United States Government and are already distributed across the United States. As remains the case with all COVID-19 vaccines, boosters will be administered without monetary cost to consumers.
|
COVID-19 Vaccination Schedule for Adults who are NOT Moderately or Severely Immunocompromised
Recommendations for adults who are not moderately or severely immunocompromised are shown below by primary series and includes 2-dose Moderna, Novavax, and Pfizer series in the first row and Janssen single-dose primary series in the second row. The recommendation has not changed; a bivalent booster continues to be recommended at least 2 months after the primary series or last monovalent booster dose. It can be either Pfizer or Moderna.
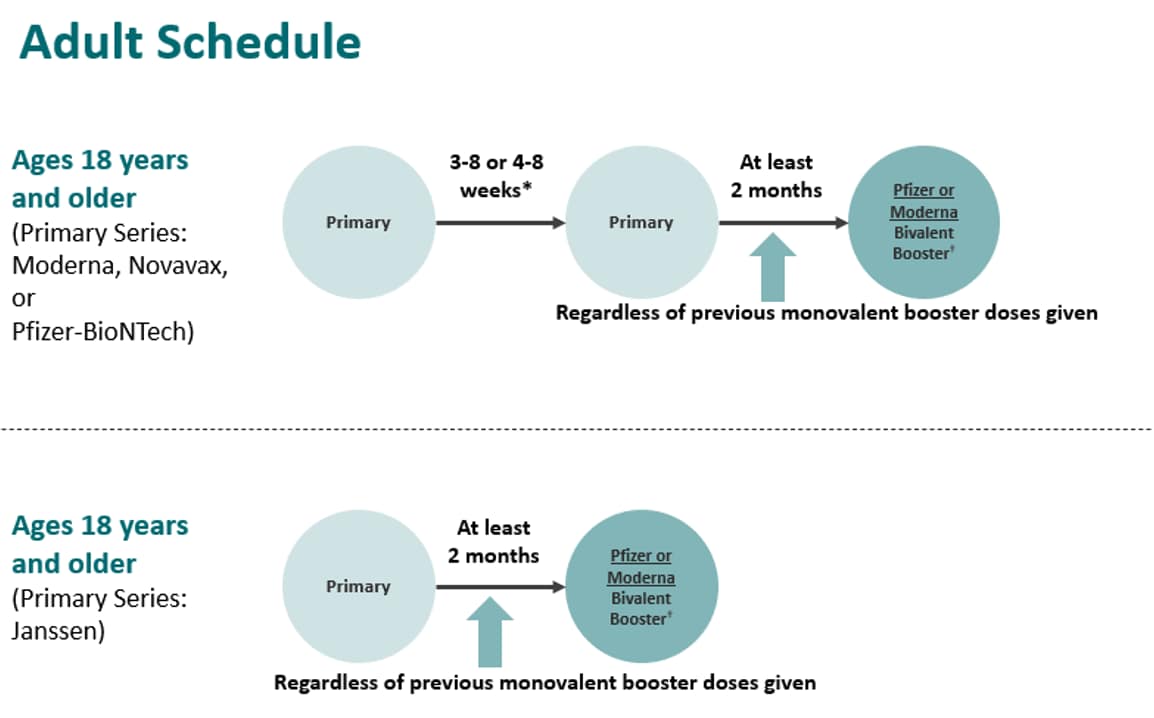
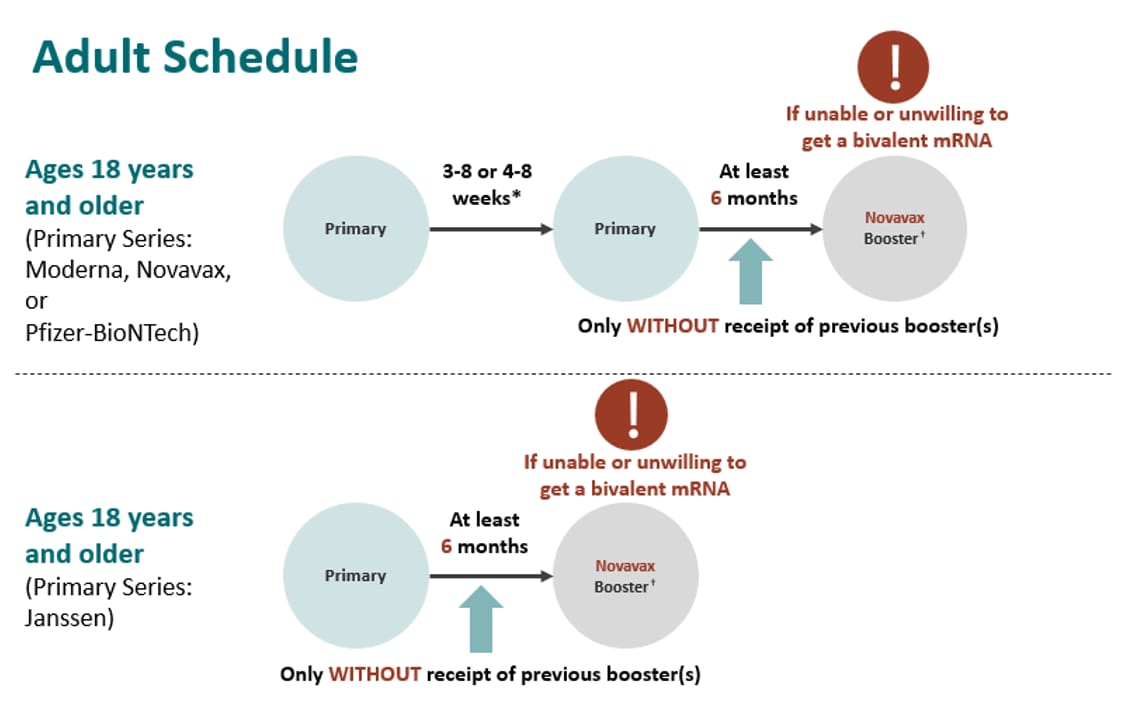
However, if adults who are not moderately or severely immunocompromised are unable or unwilling to get a bivalent mRNA booster, a monovalent Novavax booster dose is recommended at least 6 months after the primary series only without receipt of a previous booster(s).
COVID-19 Vaccination Schedule for Adults who ARE Moderately or Severely Immunocompromised
Recommendations for adults who are moderately or severely immunocompromised are shown below by primary series and includes 3-dose Moderna and Pfizer series in the first row, 2-dose Novavax in the second row, and Janssen single-dose primary series in the third row. Once again, the adult recommendation has not changed. Regardless of primary series received (the options shown in each of these rows), a bivalent booster is recommended 2 months after the primary series or last booster dose, and it can be Pfizer or Moderna.
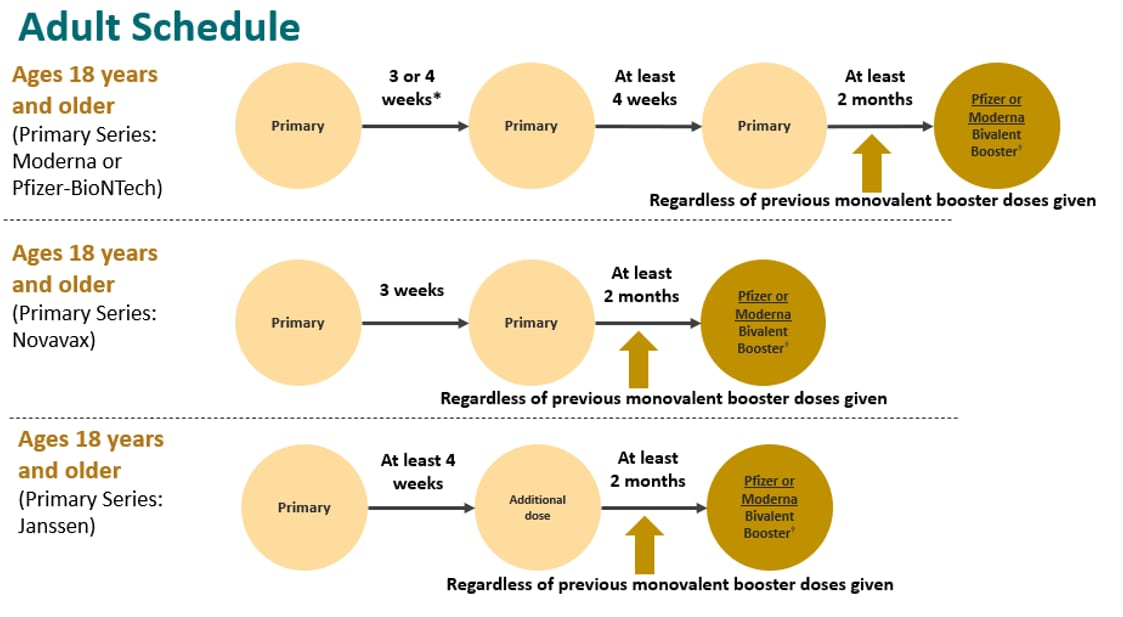
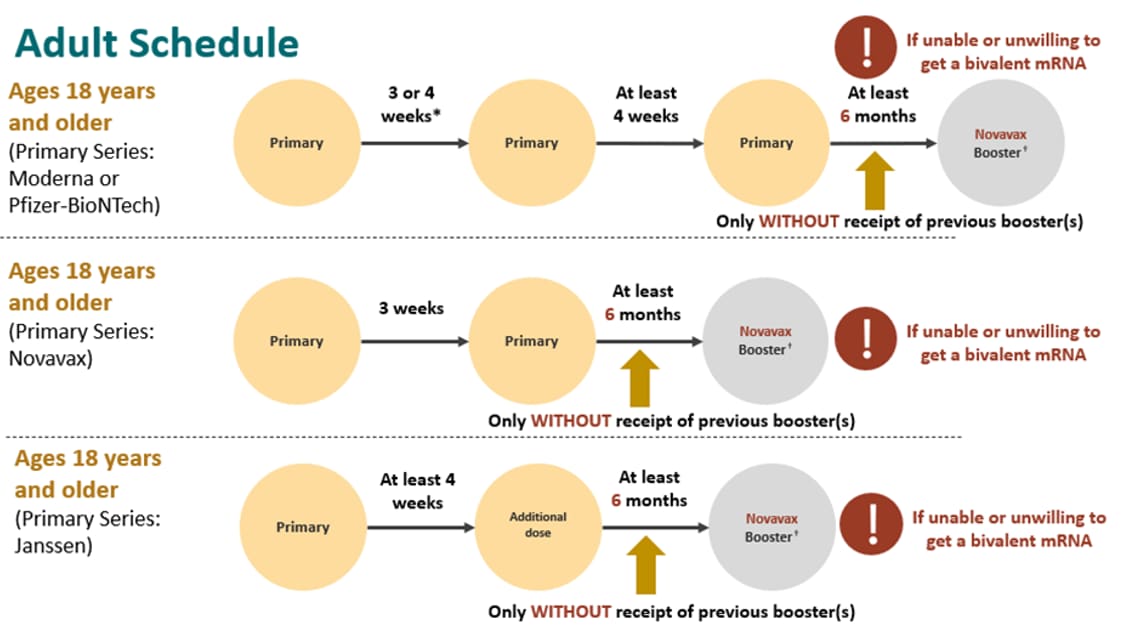
Once more, if adults who are moderately or severely immunocompromised are unable or unwilling to get a bivalent mRNA booster, a monovalent Novavax booster dose is recommended at least 6 months after the primary series only without receipt of a previous booster(s).
Novavax Booster Recommendation
A monovalent Novavax booster dose (instead of a bivalent mRNA booster dose) may be used in limited situations in people ages 18 years and older who are:
- Unable to receive an mRNA vaccine (i.e., contraindicated or in the absence of all supply)
- Unwilling to receive an mRNA vaccine and would otherwise remain unvaccinated
- Without history of prior COVID-19 booster receipt
References
Benefits and Harms:
- Novavax phase III randomized controlled trial, unpublished data from manufacturer
Values and Acceptability:
- CDC and University of Iowa/RAND survey, unpublished
Feasibility and Resource Use:
- CDC COVID Data Tracker. https://covid.cdc.gov/covid-data-tracker/#vaccinations_vacc-people-additional-dose-totalpop Accessed September 28, 2022