Targeted Testing, 2020
Table of Contents
Table 1. Persons sought, enlisted, or registered for targeted testing projects
Figure 1. Persons who sought, enlisted, or registered for targeted testing by treatment disposition
Table 4. Targeted testing evaluation indices
Table 5. Targeted testing evaluation indices, by risk type
Targeted testing is an effective prevention strategy for reducing the morbidity and mortality of tuberculosis (TB) disease in the United States. Targeted testing is used to identify and treat persons infected with M. tuberculosis. Identifying and treating persons who have latent infection (LTBI) is important as an estimated 80% of US TB cases are believed to be the result of longstanding, untreated LTBI.[1]
Beginning in 2020, CDC-funded state and city TB programs with ≥150 TB cases are required to submit targeted testing data (as outlined in the cooperative agreements). TB programs report results of their targeted testing activities to CDC by entering the data into the Aggregate Reports for Tuberculosis Program Evaluation (ARPE) form through the National Tuberculosis Indicators Project (NTIP) performance-monitoring tool. This report summarizes 2020 targeted testing data and is the first report summarizing this information.
Because targeted testing reporting includes treatment outcomes, final reporting extends over a 12-month period. For example, this means that individuals with TB infection identified in December 2020 would have until December 2021 to finish treatment, and the data would then be reported in 2022.
Targeted testing data are submitted using the following 3 categories:
Project: testing for groups done at sites outside the health department, as determined by the needs or convenience of the groups being tested. Testing projects might be held once, or they might be recurrent (e.g., annual testing at a correctional facility) or ongoing (e.g., testing of all new admissions to a homeless shelter).
Individual: testing for individuals or groups that occurs outside of testing projects; testing is often done at a health department clinic.
Administrative: testing for LTBI that is done when testing is a low public health priority because the tested persons or groups are not at risk for TB and might not be candidates for LTBI treatment. This testing is often required by regulations or policies created outside the TB control program.[2]
The number submitted in each category indicates the number of individuals the program classified under that category.
The following states and cities were required to report targeted testing data in 2020 because they had >150 TB cases in 2019: California, Georgia, Florida, Illinois, Los Angeles, Maryland, Massachusetts, New Jersey, New York, New York City, North Carolina, San Diego, Texas, Virginia, and Washington. Not all these states and cities reported or reported fully, and no TB programs with <150 cases reported targeted testing activities in 2020.”
Targeted Testing, 2020
Data shown in tables and figures are up to date as of August 2, 2022.
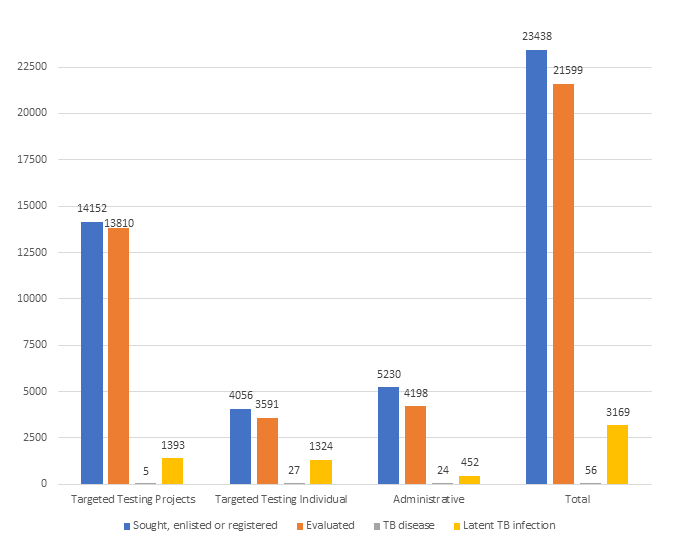
Table 1. Persons sought, enlisted, or registered for targeted testing
| Targeted testing project* | Targeted testing individual† | Administrative§ | Total | |
|---|---|---|---|---|
| Sought, enlisted, or registered | 14,152 | 4,056 | 5,230 | 23,438 |
| U.S.–born¶ | 9,936 | 96 | 201 | 10,233 |
| Non-U.S.–born¶ | 1,567 | 397 | 124 | 2,088 |
| Evaluated | 13,810 | 3,591 | 4,198 | 21,599 |
| TST¶ | 160 | >65 | 2,224 | 2,449 |
| IGRA¶ | 12,122 | 453 | 385 | 12,960 |
| TB disease | 5 | 27 | 24 | 56 |
| Latent TB infection | 1,393 | 1,324 | 452 | 3,169 |
Note. Programs reporting include California, Los Angeles, San Diego, Florida, Georgia, New Jersey, New York, North Carolina, and Virginia.
*Targeted testing project: Usually, testing projects for groups are done at sites outside the health department, as determined for the convenience or by the needs of the groups being tested. Such testing projects might be done only once during a limited period, or they might be recurrent (e.g., annual testing at a correctional facility) or ongoing (e.g., testing of all new admissions to a homeless shelter).
†Targeted testing individual: The sum of testing that is done one person at a time or by group but outside of testing projects, when testing is in accordance with national, state, or local guidelines for selecting persons who are at risk for TB and who are expected to be candidates for treatment if they have LTBI. The testing is often done at a health department clinic.
§Targeted testing administrative: Testing for LTBI that is done when testing is a low public health priority because the tested persons or groups are at low risk for TB and might not even be candidates for LTBI treatment. This testing often is required by regulations or policies created outside the TB control program.
¶Optional reporting fields.
Table 2. Persons diagnosed with latent TB infection (LTBI), by risk type and by starting and completing treatment regimen
| Targeted testing project medical risk* | Targeted testing project population risk† | Targeted testing individual medical risk* | Targeted testing individual population risk† | Administrative | Total | |
|---|---|---|---|---|---|---|
| Latent TB infection | 14 | 1,379 | 216 | 1,108 | 452 | 3,169 |
| Candidates for treatment | 12 | 1,025 | 180 | 949 | 402 | 2,568 |
| Started treatment | 12 | 451 | 140 | 622 | 253 | 1,478 |
| Completed treatment | 10 | 351 | 110 | 458 | 163 | 1,092 |
Note. Programs reporting include California, Los Angeles, San Diego, Florida, Georgia, New Jersey, New York, North Carolina, and Virginia.
* Medical risk: Persons with LTBI who have a condition that has been associated with predisposition to TB disease, usually a concurrent medical diagnosis. LTBI treatment has increased urgency for persons in this target category.
† Population risk: Persons with LTBI who are members of socially or demographically defined groups that have been associated with high prevalence of TB infection or a high transmission rate.
Table 3. Reasons patient stopped treatment for persons identified with latent TB infection (LTBI) through targeted testing
| Targeted testing project | Targeted testing individual | Administrative | Total | |
|---|---|---|---|---|
| Contacts stopping treatment (n) | 34 | 158 | 69 | 261 |
| Active TB developed | 0 | 0 | 0 | 0 |
| Adverse effect of medicine | 3 | 16 | 6 | 25 |
| Death | 0 | 0 | 1 | 1 |
| Patient chose to stop | 5 | 43 | 26 | 74 |
| Patient lost to follow-up | 25 | 92 | 33 | 150 |
| Patient moved (follow-up unknown) | 1 | 7 | 3 | 11 |
Note. Programs reporting for each project type include:
Targeted testing project: Los Angeles, San Diego, Florida, New Jersey, New York, and North Carolina.
Targeted testing individual: Florida, New York, North Carolina
Administrative: Florida, New York, North Carolina
Evaluation Indices, 2020
Table 4. Targeted testing evaluation indices
| Targeted testing project | Targeted testing individual | Administrative | |
|---|---|---|---|
| Evaluation % | 97.6 | 88.5 | 80.3 |
| Disease % | 0.0 | 0.8 | 0.6 |
| Latent TB infection % | 10.1 | 36.9 | 10.8 |
Note. Programs reporting include California, Los Angeles, San Diego, Florida, Georgia, New Jersey, New York, North Carolina, and Virginia.
Table 5. Targeted testing evaluation indices, by risk type
| Targeted testing project medical risk* | Targeted testing project population risk† | Targeted testing individual medical risk* | Targeted testing individual population risk† | Administrative | |
| Candidate % | 85.7 | 74.3 | 83.3 | 85.6 | 88.9 |
| Treatment % | 100 | 44.0 | 77.8 | 65.5 | 62.9 |
| Completion% | 83.3 | 77.8 | 78.6 | 73.6 | 64.4 |
Note. Programs reporting include California, Los Angeles, San Diego, Florida, Georgia, New Jersey, New York, North Carolina, and Virginia.
* Medical risk: Persons with LTBI who have a condition that has been associated with predisposition to TB disease, usually a concurrent medical diagnosis. LTBI treatment has increased urgency for persons in this target category.
† Population risk: Persons with LTBI who are members of socially or demographically defined groups that have been associated with high prevalence of TB infection or a high transmission rate