2020 Contact Investigations Report (ARPE Data)
Table of Contents
Table 1: Counts of Sputum AFB Smear-Positive Cases and Contacts
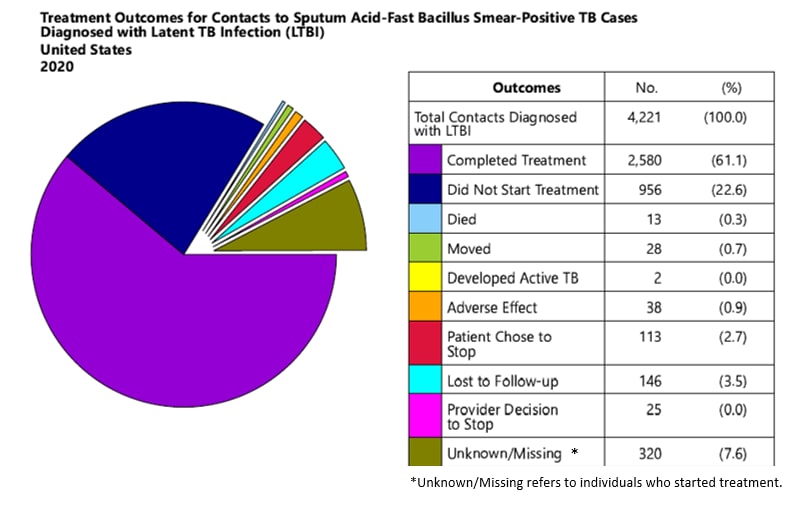
Table 2: Reasons Contacts Stopped Latent TB Infection Treatment, Sputum AFB Smear-Positive Cases
Table 3: Contact Investigation Evaluation Indices, Sputum AFB Smear-Positive Cases
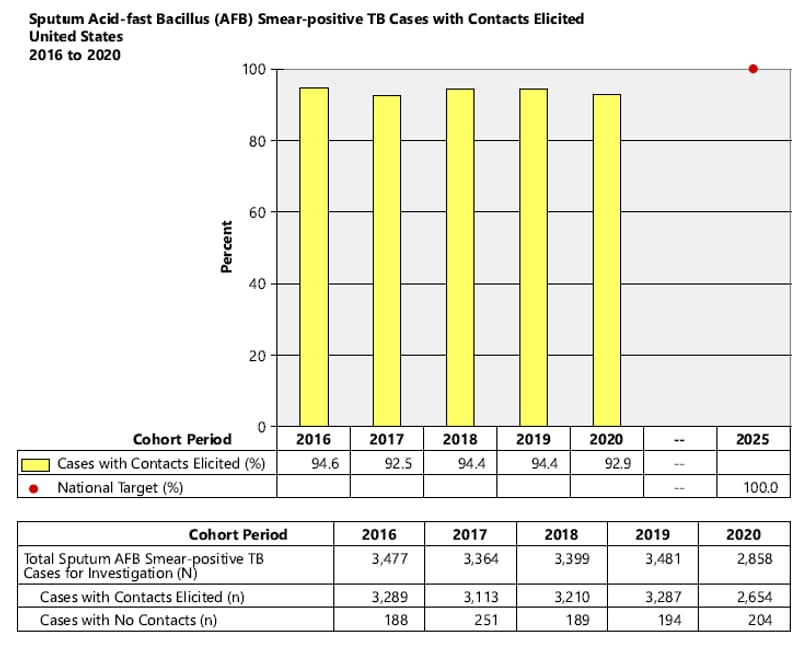
- National Objective: Increase the proportion of TB patients with positive acid-fast bacillus (AFB) sputum-smear results who have contacts elicited to 100.0% by 2025
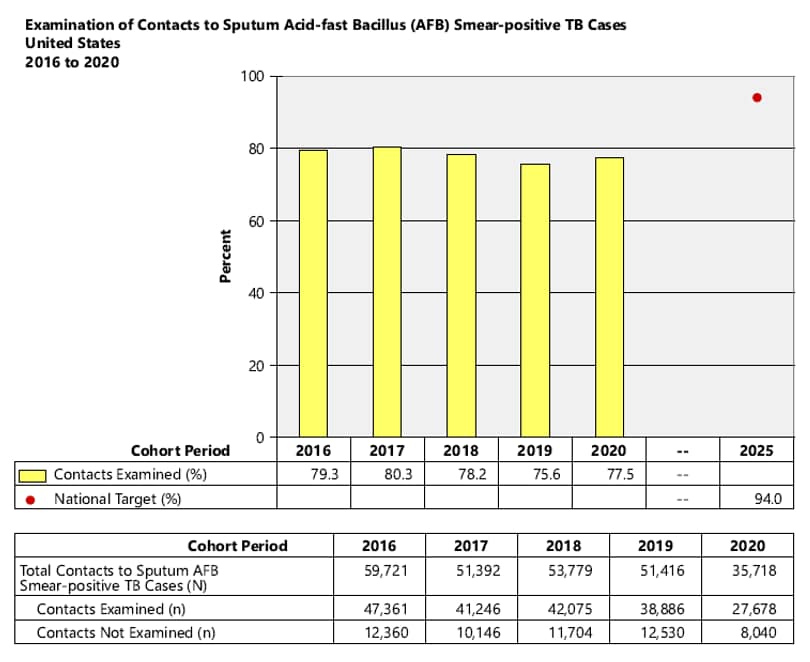
- National Objective: Increase the proportion of contacts to sputum acid-fast bacillus (AFB)smear-positive TB cases who are examined for infection and disease to 94.0% by 2025
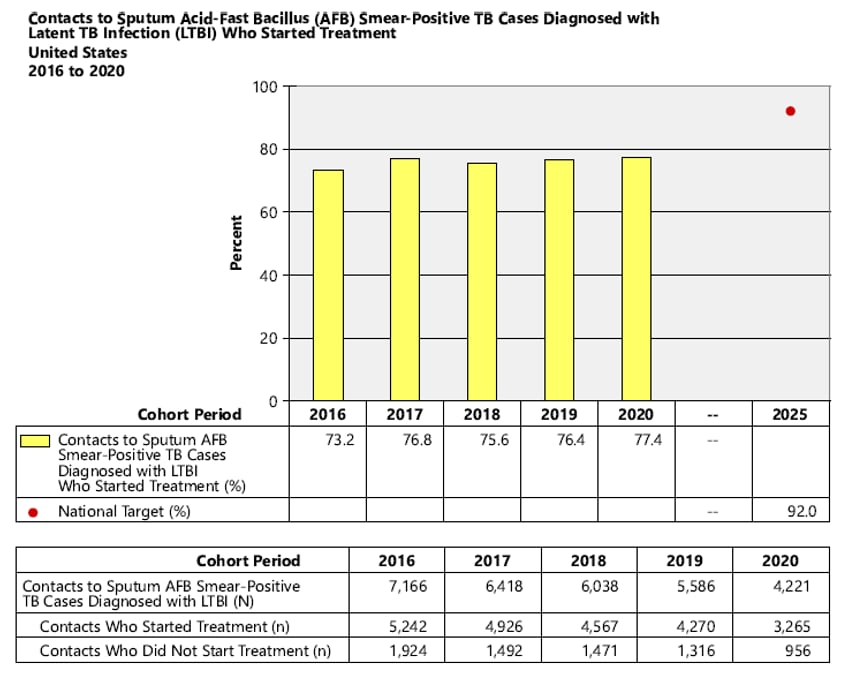
- National Objective: Increase the proportion of contacts to sputum acid-fast bacillus (AFB) smear-positive TB cases diagnosed with latent TB Infection (LTBI) who start treatment to 92.0% by 2025
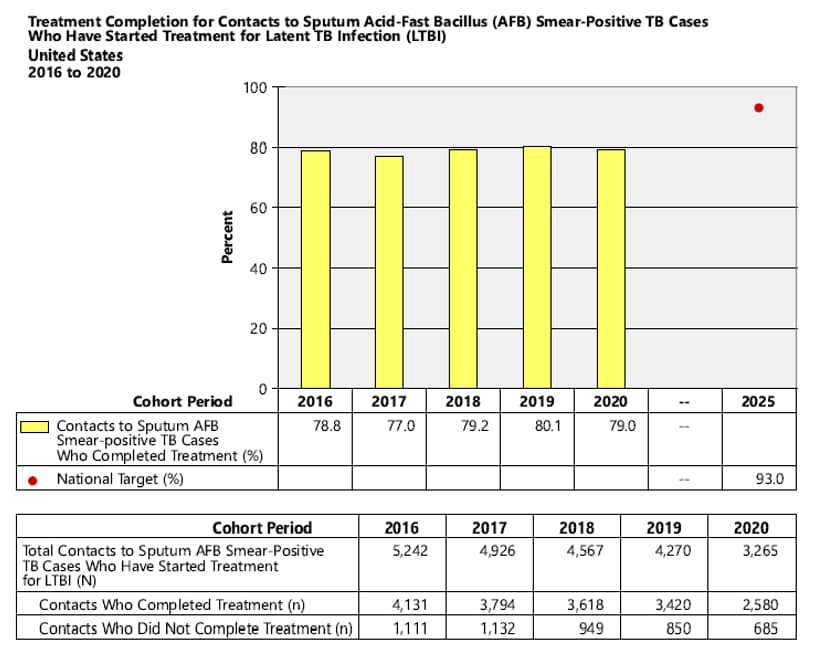
- National Objective: For contacts to sputum acid-fast bacillus (AFB) smear-positive TB cases who have started treatment for latent TB infection (LTBI), increase the proportion who complete treatment to 93.0% by 2025
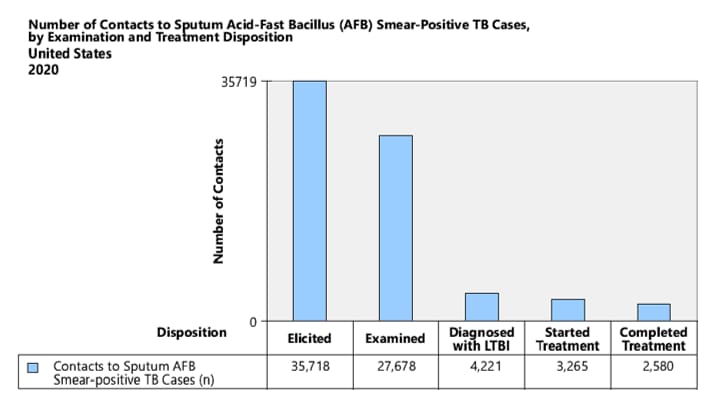
- Number of Contacts to Sputum acid-fast bacillus (AFB) Smear-Positive TB Cases
Table 4: Counts of Sputum AFB Smear-Negative, Culture-Positive Cases and Contacts
Table 6: Contact Investigation Evaluation Indices Sputum AFB Smear-Negative, Culture-Positive Cases
Appendix C. Counts and Indices for Investigation of Other* Cases, 2017-2021
Table 7: Counts of Contacts, Other Cases
Table 8: Reasons Contacts Stopped Treatment for Latent TB Infection, Other Cases
Table 9: Contact Investigation Evaluation Indices, Other Cases
Appendix A. Counts and Indices for Investigation of Sputum Acid-fast Bacillus (AFB) Smear-Positive TB Cases, 2017-20211
Table 1: Counts of Smear-Positive Cases and Contacts
| Cases and Contacts | 2017 | 2018 | 2019 | 2020 | 2021 |
|---|---|---|---|---|---|
| Cases for investigation | 3364 | 3398 | 3481 | 2879 | 3320 |
| Cases, no contacts | 247 | 187 | 193 | 206 | 270 |
| Number of contacts | 51447 | 53783 | 51419 | 35915 | 27202 |
| Evaluated | 41489 | 42302 | 38942 | 28095 | 20738 |
| TB disease | 416 | 352 | 345 | 317 | 381 |
| Latent TB infection | 6428 | 6044 | 5587 | 4229 | 4438 |
| Started treatment | 4952 | 4583 | 4271 |
3290 |
3272 |
| Completed treatment | 3804 | 3623 | 3423 | 2600 | 2657 |
| Treatment outcome unknown/missing | 231 | 366 | 283 | 335 | 263 |
Table 2: Reasons Contacts Stopped LTBI Treatment, Smear-Positive Cases
| Reason | 2016 | 2017 | 2018 | 2019 | 2020 |
| Contacts stopping treatment (n) | 873 | 908 | 595 | 563 | 365 |
| Active TB developed | 17 (2%) | 7 (1%) | 5 (<1%) | 4 (1%) | 2 (<1%) |
| Adverse effect of medicine | 103 (12%) | 53 (6%) | 48 (8%) | 47 (8%) | 38 (10%) |
| Contact chose to stop | 335 (38%) | 299 (33%) | 257 (43%) | 188 (33%) | 113 (31%) |
| Contact lost to follow-up | 291 (33%) | 414 (46%) | 193 (33%) | 248 (44%) | 146 (40%) |
| Contact moved (follow-up unknown) | 62 (7%) | 67 (7%) | 37 (6%) | 38 (7%) | 28 (8%) |
| Death | 5 (1%) | 6 (1%) | 4 (1%) | 7 (1%) | 13 (4%) |
| Provider decision | 60 (7%) | 62 (7%) | 51 (9%) | 31 (6%) | 25 (7%) |
Table 3: Contact Investigation Evaluation Indices, Smear-Positive Cases
| Evaluation Indices (%) | 2016 | 2017 | 2018 | 2019 | 2020 |
| Contact Elicitation (%) | 94.6% | 92.5% | 94.4% | 94.4% | 92.9% |
| Contacts Per Case (N, Average) | 17.2 | 15.3 | 15.8 | 14.8 | 12.5 |
| Contact Evaluation (%) | 79.3% | 80.3% | 78.2% | 75.6% | 77.5% |
| TB Disease (%) | 0.7% | 1.0% | 0.8% | 0.9% | 1.1% |
| Latent TB Infection (%) | 15.1% | 15.6% | 14.4% | 14.4% | 15.3% |
| LTBI Treatment Initiated (%) | 73.2% | 76.8% | 75.6% | 76.4% | 77.4% |
| LTBI Treatment Completed (%) | 78.8% | 77.0% | 79.2% | 80.1% | 79.0% |
1Reporting jurisdictions: 50 U.S. states, Washington D.C., Puerto Rico and eight large city jurisdictions (Los Angeles, CA, San Francisco, CA, San Diego, CA, Chicago, IL, Baltimore, MD, New York City, NY, Philadelphia, PA, Houston, TX). Missouri did not report in 2017 and 2018, and Kansas did not report in 2016 -2018. Nebraska, and Virgin Islands did not report in 2018. Vermont did not report in 2019. Iowa did not report in 2020. Data shown in tables and figures are up to date as of July 11, 2022
Figures
1. National Objective: Increase the proportion of TB patients with positive acid-fast bacillus (AFB) sputum-smear results who have contacts elicited to 100.0% by 2025.
Objective: Increase the proportion of TB patients with positive acid-fast bacillus (AFB) sputum-smear results who have contacts elicited to 100.0% by 2025.
Indicator: Percent of sputum AFB smear-positive TB cases with contacts elicited.
Cohort: Sputum AFB smear-positive TB cases counted in the cohort period of interest. Records with missing or incomplete data where exclusion criteria cannot be assessed are included in the analytic cohort.
Data Sources: ARPEs (Contacts) fields, a1 (Sputum smear+ Cases for Investigation), b1 (Sputum smear+ Cases with No Contacts).
Sputum AFB smear-positive TB Cases with contacts elicited = Number of sputum AFB smear-positive TB cases for investigation – Number of sputum AFB smear-positive TB cases with no contacts.
Calculation: [(Number of sputum AFB smear-positive TB cases with contacts elicited) / Cohort] x 100.
2. National Objective: Increase the proportion of contacts to sputum AFB smear-positive TB cases who are examined for infection and disease to 94.0% by 2025.
Objective: Increase the proportion of contacts to sputum AFB smear-positive TB cases who are examined for infection and disease to 94.0% by 2025.
Contacts are counted as evaluated if they have been tested and examined to the point where a final determination of diagnosis is either latent TB infection or TB disease.
Indicator: Percent of contacts to sputum AFB smear-positive TB cases examined for infection and disease.
Cohort: Contacts to sputum AFB smear-positive TB cases counted in the cohort period of interest. Records with missing or incomplete data where exclusion criteria cannot be assessed are included in the analytic cohort.
Data Sources: ARPEs (Contacts) fields, c1 (Number of Contacts to Sputum smear+ Cases), d1 (Contacts to Sputum smear+ Cases Evaluated).
Calculation: [Number of contacts to sputum AFB smear-positive TB cases evaluated / Cohort] x 100.
3. National Objective: Increase the proportion of contacts to sputum AFB smear-positive TB cases diagnosed with latent TB infection (LTBI) who start treatment to 92.0% by 2025.
Objective: Increase the proportion of contacts to sputum acid-fast bacillus (AFB) smear-positive TB cases diagnosed with latent TB infection (LTBI) who start treatment to 92.0% by 2025.
Indicator: Percent of contacts to sputum AFB smear-positive TB cases diagnosed with LTBI who started treatment NOTE: Contacts are counted as having started treatment after taking the first dose of a treatment course.
Cohort: Contacts to sputum AFB smear-positive TB cases who have newly diagnosed LTBI, counted in the cohort period of interest. Records with missing or incomplete data where exclusion criteria cannot be assessed are included in the analytic cohort.
Data Sources: ARPEs (Contacts) fields, f1 (Contacts to Sputum smear+ Cases with Latent TB Infection), g1 (Contacts to Sputum smear+ Cases Diagnosed with Latent TB Infection Who Started Treatment).
Calculation: [Number of contacts diagnosed with LTBI who started treatment / Cohort] x 100.
4. National Objective: For contacts to sputum AFB smear-positive TB cases who have started treatment for LTBI, increase the proportion who complete treatment to 93.0% by 2025.
Objective: For contacts to sputum AFB smear-positive TB cases who have started treatment for LTBI, increase the proportion who complete treatment to 93.0% by 2025.
Indicator: Percent of contacts to sputum AFB smear-positive TB cases who completed treatment.
Cohort: Contacts to sputum AFB smear-positive TB cases with newly diagnosed LTBI and have started treatment, counted in the cohort period of interest. Records with missing or incomplete data where exclusion criteria cannot be assessed are included in the analytic cohort.
Data Sources: ARPEs (Contacts) fields, g1 (Contacts to Sputum smear+ Cases Diagnosed with Latent TB Infection Who Started Treatment), h1 (Contacts to Sputum smear+ Cases Diagnosed with Latent TB Infection Who Completed Treatment).
Calculation: [Number of contacts diagnosed with LTBI who completed treatment / Cohort] x 100.
Appendix B. Counts and Indices for Investigation of Sputum AFB Smear-Negative, Culture-Positive TB Cases, 2016-20202
Table 4: Counts of Smear-Negative, Culture-Positive Cases and Contacts
| Cases and Contacts | 2016 | 2017 | 2018 | 2019 | 2020 |
| Cases for investigation | 1,932 | 1,827 | 1,903 | 1,784 | 1333 |
| Cases, no contacts | 401 | 312 | 258 | 257 | 205 |
| Number contacts | 13,914 | 14,100 | 13,589 | 12,679 | 8795 |
| Evaluated | 11,460 | 11,627 | 10,703 | 9,905 | 6893 |
| TB disease | 60 | 72 | 72 | 63 | 66 |
| Latent TB infection | 1,493 | 1,319 | 1,427 | 1,146 | 896 |
| Started treatment | 965 | 889 | 959 | 746 | 562 |
| Completed treatment | 765 | 702 | 744 | 606 | 407 |
| Treatment outcome unknown/missing | 4 | 44 | 43 | 32 | 92 |
Table 5: Reasons Contacts Stopped LTBI Treatment – Smear-Negative, Culture-Positive Cases
| Reason | 2016 | 2017 | 2018 | 2019 | 2020 |
| Contacts stopping treatment (n) | 196 | 143 | 172 | 108 | 63 |
| Active TB developed | 1 (1%) | 1 (1%) | 4 (2%) | 2 (2%) | 0 (0%) |
| Adverse effect of medicine | 22 (11%) | 17 (12%) | 18 (10%) | 13 (12%) | 12 (19%) |
| Contact chose to stop | 79(40%) | 49 (34%) | 71 (41%) | 38 (35%) | 25 (40%) |
| Contact lost to follow-up | 50 (26%) | 44 (31%) | 55 (32%) | 42 (39%) | 19 (30%) |
| Contact moved (follow-up unknown) | 20 (10%) | 15 (10%) | 10 (6%) | 6 (6%) | 3 (5%) |
| Death | 2 (1%) | 1 (1%) | 2 (1%) | 4 (4%) | 2 (3%) |
| Provider decision | 22 (11%) | 16 (11%) | 12 (7%) | 3 (3%) | 2 (3%) |
Table 6: Contact Investigation Evaluation Indices – Smear-Negative, Culture-Positive Cases
| Evaluation Indices (%) | 2016 | 2017 | 2018 | 2019 | 2020 |
| Contact Elicitation (%) | 79.2% | 82.9% | 86.4% | 85.6% | 84.6% |
| Contacts Per Case (N, Average) | 7.2 | 7.7 | 7.1 | 7.1 | 6.6 |
| Contact Evaluation (%) | 82.4% | 82.5% | 78.8% | 78.1% | 78.4% |
| TB Disease (%) | 0.5% | 0.6% | 0.7% | 0.6% | 0.9% |
| Latent TB Infection (%) | 13.0% | 11.3% | 13.3% | 11.6% | 12.9% |
| LTBI Treatment Initiated (%) | 64.6% | 67.4% | 67.2% | 65.1% | 62.7% |
| LTBI Treatment Completed (%) | 79.3% | 78.9% | 77.6% | 81.2% | 72.4% |
2Reporting jurisdictions: 50 U.S. states, Washington D.C., Puerto Rico and eight large city jurisdictions (Los Angeles, CA, San Francisco, CA, San Diego, CA, Chicago, IL, Baltimore, MD, New York City, NY, Philadelphia, PA, Houston, TX). Missouri did not report in 2017 and 2018, and Kansas did not report in 2016 -2018. Nebraska, and Virgin Islands did not report in 2018. Vermont did not report in 2019. Iowa did not report in 2020. Data shown in tables and figures are up to date as of July 11, 2022.
Appendix C. Counts and Indices for Investigation of Other* Cases, 2016-20203
* Other = Contact investigations not captured in investigating sputum AFB smear-positive or sputum AFB smear-negative\culture-positive. For example: Associate-contact or source-case investigations done because of a child with TB.
Table 7: Counts of Contacts, Other Cases
| Contacts | 2016 | 2017 | 2018 | 2019 | 2020 |
| Number contacts | 8,119 | 7,936 | 10,656 | 8,284 | 5605 |
| Evaluated | 6,648 | 6,760 | 8,810 | 6,170 | 4271 |
| TB disease | 52 | 62 | 68 | 66 | 67 |
| Latent TB infection | 847 | 721 | 821 | 795 | 509 |
| Started treatment | 587 | 531 | 574 | 549 | 336 |
| Completed treatment | 454 | 427 | 492 | 455 | 276 |
| Unknown/Missing | 17 | 25 | 15 | 25 | 12 |
Table 8: Reasons Contacts Stopped Treatment for LTBI, Other Cases
| Reason | 2016 | 2017 | 2018 | 2019 | 2020 |
| Contacts stopping treatment (n) | 116 | 79 | 67 | 71 | 48 |
| Active TB developed | 3 (3%) | 4 (5%) | 2 (3%) | 0 (0%) | 0 (0%) |
| Adverse effect of medicine | 11 (10%) | 5 (6%) | 9 (13%) | 14 (20%) | 6 (13%) |
| Contact chose to stop | 46 (40%) | 31 (39%) | 18 (27%) | 27 (38%) | 18 (38%) |
| Contact lost to follow-up | 27 (23%) | 26 (33%) | 26 (39%) | 21 (30%) | 18 (38%) |
| Contact moved (follow-up unknown) | 18 (16%) | 8 (10%) | 5 (7%) | 6 (8%) | 4 (8%) |
| Death | 0 (0%) | 0 (0%) | 1 (2%) | 0 (0%) | 0 (0%) |
| Provider decision | 11 (9%) | 5 (6%) | 6 (9%) | 3 (4%) | 2 (4%) |
Table 9: Contact Investigation Evaluation Indices, Other Cases
| Evaluation Indices (%) | 2016 | 2017 | 2018 | 2019 | 2020 |
| Contact Evaluation (%) | 81.9% | 85.2% | 82.7% | 74.5% | 76.2% |
| TB Disease (%) | 0.8% | 0.9% | 0.8% | 1.1% | 1.6% |
| Latent TB Infection (%) | 12.7% | 10.7% | 9.3% | 12.9% | 11.9% |
| LTBI Treatment Initiated (%) | 69.3% | 73.6% | 69.9% | 69.1% | 66.0% |
| LTBI Treatment Completed (%) | 77.3% | 80.4% | 85.7% | 82.9% | 82.1% |
3Reporting jurisdictions: 50 U.S. states, Washington D.C., Puerto Rico and eight large city jurisdictions (Los Angeles, CA, San Francisco, CA, San Diego, CA, Chicago, IL, Baltimore, MD, New York City, NY, Philadelphia, PA, Houston, TX). Missouri did not report in 2017 and 2018, and Kansas did not report in 2016 -2018. Nebraska, and Virgin Islands did not report in 2018. Vermont did not report in 2019. Iowa did not report in 2020. Data shown in tables and figures are up to date as of July 11, 2022.