Disk Diffusion Susceptibility Testing
Testing is performed according to Clinical and Laboratory Standards Institute (CLSI)*-recommended procedures. Laboratories performing susceptibility testing by non-CLSI procedures should use quality control and interpretive criteria appropriate for the specific method used. *formerly National Committee for Clinical Laboratory Standards (NCCLS)
NEISSERIA GONORRHOEAE REFERENCE STRAINS FOR ANTIMICROBIAL SUSCEPTIBILITY TESTING
INTENDED USE
Reference strains listed in this brochure are quality control (QC) strains for the agar-dilution, disk diffusion, and Etest susceptibility procedures used to determine the antimicrobial susceptibilities of isolates of Neisseria gonorrhoeae. These strains may be used to assess the quality of susceptibilities of N. gonorrhoeae isolates to penicillin, tetracycline, spectinomycin, ceftriaxone, cefixime, ciprofloxacin, ofloxacin, levofloxacin, and azithromycin. It is intended that these strains be used in conjunction with strain ATCC 49226 as recommended by the Clinical and Laboratory Standards Institute (CLSI)* for QC of susceptibility testing of gonococcal isolates (1, 2).
*formerly National Committee for Clinical Laboratory Standards (NCCLS)
ANTIMICROBIAL RESISTANCE IN N. GONORRHOEAE
N. gonorrhoeae usually develops resistance to antimicrobial agents within a few years of their introduction for gonorrhea therapy. Antimicrobial resistance in N. gonorrhoeae occurs as chromosomally mediated resistance to a variety of antimicrobial agents, including penicillin, tetracycline, spectinomycin, and fluoroquinolones (3, 4), and high-level, plasmid-mediated resistance to penicillin and tetracycline (3).
Sentinel surveillance to monitor antimicrobial resistance in N. gonorrhoeae in the United States is performed by the Gonococcal Isolate Surveillance Project (GISP), sponsored by the Centers for Disease Control and Prevention (CDC) in collaboration with local health departments. In GISP, antimicrobial susceptibilities of N. gonorrhoeae isolated from men in approximately 26 cities in the United States are determined (5); annual reports for GISP may be obtained electronically from the internet (6). Because of the spread of gonococcal isolates resistant to penicillin and tetracycline, CDC has recommended that extended-spectrum cephalosporins (ceftriaxone, cefixime) and fluoroquinolones (ciprofloxacin, ofloxacin, levofloxacin) be used as primary therapies to treat uncomplicated gonorrhea (7). At this time, no failures of gonococcal infections to respond to cephalosporins have been confirmed. Failures of gonococcal infections to respond to fluoroquinolones (ciprofloxacin, ofloxacin) have been reported. Fluoroquinolone-resistant isolates are very prevalent in Asia and have been isolated with increasing frequency in the United States. In response to this increase, many states have revised recommendations for the treatment of gonorrhea (8).
Agar dilution susceptibility testing is the “gold standard” for susceptibility testing of N. gonorrhoeae. However, when performed correctly, the disk-diffusion and Etest susceptibility tests can be used to identify isolates of N. gonorrhoeae that exhibit decreased susceptibility, intermediate resistance, and resistance to antimicrobial agents.
DISCLAIMER
This brochure contains current CLSI-recommended procedures and interpretive criteria for agents used for the treatment of uncomplicated gonorrhea and for routine surveillance of antimicrobial resistance in N. gonorrhoeae (1, 2). The CLSI may revise interpretive criteria contained in this brochure. Consumers in the United States are advised always to consult current CLSI publications and to use the recommended methods and interpretive criteria contained in current CLSI documents if they differ from those in this brochure to ensure compliance with the requirements of the Clinical Laboratory Improvement Amendments (CLIA). Consumers are also advised that results obtained with procedures, media, etc. other than those described in CLSI documents may not give results comparable to those obtained with the CLSI-recommended procedures for QC isolate ATCC 49226 or for CDC-recommended QC strains. If results do not agree with those in CLSI documents (1, 2), it is not appropriate to interpret such results using CLSI-recommended interpretive criteria.
PRINCIPLES OF THE PROCEDURE
In the disk-diffusion susceptibility test, disks containing known amounts of an antimicrobial agent are placed on the surface of an agar plate containing a nonselective medium that has been inoculated with a suspension of a strain of N. gonorrhoeae to give a confluent lawn of growth. The antimicrobial agent diffuses into the medium, causing a zone of inhibition of growth of the strain around the disk corresponding to the susceptibility of the strain to the agent. Interpretative inhibition zone diameters have been established for susceptibility test results to permit classification of an isolate as being susceptible, intermediate (or exhibiting decreased susceptibility), or resistant to an antimicrobial agent (Table 1; 1, 2).
Note: The Etest is performed using a procedure similar to that described in this brochure. The Etest is a strip containing a known gradient of an antimicrobial agent and calibrated to give results as minimal inhibitory concentrations (MICs) of the agents. Etest strips are placed on the surface of a 150-mm plate (containing a nonselective medium inoculated as in the disk diffusion method) in a radial pattern with the lowest concentration of the agent toward the center of the plate and the highest concentration of the agent toward the edge of the plate). If MICs for ATCC 49226 correspond with CLSI-designated QC values, MICs of clinical isolates may be interpreted according to CLSI interpretive criteria. Consult Etest product literature for complete instructions.
REFERENCE STRAINS
Gonococcal antimicrobial susceptibility reference strains include strain ATCC 49226, which is the CLSI-designated QC strain for antimicrobial susceptibility testing of N. gonorrhoeae. Additional QC strains are used at CDC to control for resistance to penicillin, tetracycline, and spectinomycin; intermediate resistance and resistance to ciprofloxacin and ofloxacin; high MICs of azithromycin and levofloxacin (MICs ≥1.0 µg/ml); and decreased susceptibility to cefixime. It is intended that these strains be used in conjunction with the CLSI-recommended strain ATCC 49226 when determining the susceptibilities of gonococcal isolates (1, 2). Strains have been grown in large volumes, dispensed in 0.5-ml volumes, lyophilized, and stored at 4 C. The strains exhibit the MICs and zone inhibition diameters or modal MICs shown in Table 2a and Table 2b.
RECOVERY, MAINTENANCE, AND STORAGE OF REFERENCE STRAINS
- Re-hydrate the strains in approximately 0.5 ml of trypticase soy broth containing 20% glycerol (freezing medium for N. gonorrhoeae), Mueller-Hinton broth, or sterile saline; phosphate buffered saline (pH 7.2) may also be used.
- Inoculate the suspension of re-hydrated organisms onto plates of a selective medium by the streak plate method to allow growth of individual colonies. Examine plates for growth after incubation at 35 C to 36.5 C in 5% carbon dioxide for 24 to 48 h.
- Subculture isolated colonies resembling N. gonorrhoeae onto a nonselective medium (e.g., chocolate agar) and incubate plates at 35 C to 36.5 C in 5% carbon dioxide for 24 h. Confirm that the culture is pure and composed only of gram-negative, oxidase-positive diplococci. (Although sample vials of each strain have been tested, the strain may have been contaminated during preparation or re-hydration.) It is also advisable to confirm the identification of the organism.
- To store strains for future use, prepare a dense suspension with growth from a 24-h pure culture (on a nonselective medium) in 0.5- to 1.0-ml trypticase soy broth containing 20% glycerol. Strains should be stored at -70 C or in liquid nitrogen. It is not advisable to store gonococcal isolates at -20 C because they may rapidly lose viability at this temperature.Note: Cultures for susceptibility testing must be subcultured every 24 h or prepared from a frozen stock culture every week of use and subcultured every 24 h until testing is performed. Do not incubate cultures for susceptibility testing for 48 h; suspensions should be prepared from cultures incubated for 18 to 24 h. Do not use 24-h cultures of strains from the frozen preparation for susceptibility testing; strains must be subcultured at least once after recovery from a frozen suspension before being used to prepare suspensions for susceptibility testing.
MATERIALS
- Gonococcal reference strain ATCC 49226 (F-18) and additional QC strains as required
- Selective medium (Martin-Lewis, modified Thayer-Martin, GC-Lect, or equivalent medium)
- Nonselective medium (chocolate agar supplemented with 1% IsoVitaleX or an equivalent medium)
- Sterile trypticase soy broth containing 20% glycerol, Mueller-Hinton broth, saline, or phosphate buffered saline, pH 7.2
- Pasteur pipettes and rubber bulb or tuberculin syringes with 27-gauge needles
- Microbiological loop
- Incubator at 35 C to 36.5 C; use CO2 incubator or candle extinction jars to provide supplemental use CO2
- GC base medium containing 1% (v/v) IsoVitaleX or equivalent supplement (60 ml of medium in 150-mm Petri dishes) for disk diffusion testing
- Antibiotic disks or Etest strips
- 0.5 McFarland barium sulfate [BaSO4] standard (McFarland Turbidity Standard)
DISK-DIFFUSION TEST PROCEDURE
- Warm, to room temperature, the appropriate number of GC agar plates for each strain to be tested.Note 1: The number of plates required per strain tested will depend on the anticipated zone inhibition diameters of the antimicrobial agents to be tested; disks should be placed on plates so that the zones of inhibition do not overlap. It may be necessary to experiment to determine the best combination and placement of disks to minimize the number of plates required.Note 2: The surface of the plates should be dry before culture suspensions are inoculated. If moisture is visible on the surface of the plates, dry them (with lids ajar) in a 35 C incubator just prior to inoculation. There should be no visible droplets of moisture on the surface of the agar or on the lids of the plates when they are inoculated. The surface of the dried medium should be smooth and should not show signs (webbed ribbing pattern on the agar surface) of desiccation.
- Suspend isolated colonies from an overnight culture on supplemented chocolate agar medium in 1.0 to 2.0 ml of Mueller-Hinton broth. Mix the suspension thoroughly on a vortex mixer to break up clumps of growth.
- Adjust the turbidity of the cell suspension by adding additional Mueller-Hinton broth or organisms, as required, until the turbidity of the suspension is equivalent to the turbidity of a 0.5 McFarland BaSO4 standard. Discard this cell suspension if it is not used within 15 to 20 min after preparation, and prepare a fresh suspension for testing.
- Moisten a sterile applicator swab in the standardized cell suspension, and express excess moisture by rotating the swab against the glass above the liquid in the tube. Inoculate the entire surface of each plate, inoculating the surface completely in three different directions to ensure uniform, confluent growth (Figure 1).Figure 1. The entire surface of the plate is inoculated in three directions as indicated to ensure uniform growth
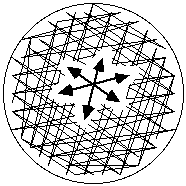
Note 1: It is recommended that cotton swabs with wooden handles be used for this procedure. Synthetic swabs do not soak up sufficient suspension to inoculate the entire surface of the plate.
Note 2: It is recommended that swabs with plastic handles not be used to inoculate plates; the handles bend and may splatter liquid out of the tube when excess suspension is being expressed, creating a biohazard.
- Repeat step 4, using a new sterile swab, to inoculate each additional plate as needed.
- Allow the inoculated plates to sit at room temperature for 3 to 5 min to allow the moisture from the inoculum to absorb into the medium. Inspect the inoculated plates to ensure that there is no visible liquid on the surface of the medium; the surface of the medium must be dry before the disks are applied.
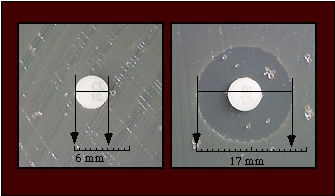
- When the surface of the medium is dry, apply disks of the selected antimicrobial agents to the surface of the medium and tamp them gently with a sterile loop or forceps to ensure that they are in complete contact with the agar surface. All disks should be applied approximately the same distance from the edge of the plate and from each other (Figure 2).Figure 2. Antibiotic disks are placed approximately the same distance from the edge of the plate and from each other. Ensure that the disks are in complete contact with the surface of the agar; tamp with a sterile loop or forceps if necessary. The zones of inhibition are measured as shown in the figure.
- Invert the inoculated plates (lid side down), and incubate the plates at 35 C to 36 C in 5% CO2 for 20 to 24 h.
- Examine the disk diffusion plates from the back, viewed against a black background and illuminated with reflected light. With a caliper, measure and record the diameter of each zone of inhibition to the nearest whole millimeter.
CLSI recommends a range of values for the susceptibility of N. gonorrhoeae strain ATCC 49226 to a variety of antimicrobial agents (1). The values given for ATCC 49226 for azithromycin and for all antimicrobial agents for other reference strains in this protocol were obtained when strains were tested on GC II agar base supplemented with 1% IsoVitaleX (Table 2a and Table 2b) and determined in the Neisseria Reference Laboratory (NRL) at CDC and at five GISP regional laboratories located at Emory University, Atlanta, Georgia; University of Washington, Seattle, Washington; University of Alabama, Birmingham, Alabama; University of Colorado, Denver, Colorado; The Cleveland Clinic Foundation, Cleveland, Ohio (5). Values may vary slightly when strains are tested on other media.
If the results for reference strain ATCC 49226 meet those recommended by NCCLS and those listed in Table 2a or Table 2b for additional QC strains, the results for the test strains may be interpreted according to the criteria listed in Table 1.
LIMITATIONS OF THE TEST
The MICs and inhibition zone sizes given in this protocol for ATCC 49226 are recommended by CLSI, MIC results for additional QC isolates are derived from testing performed at the NRL, CDC and in the GISP regional laboratories, and disk diffusion inhibition zone diameters were determined at the NRL, CDC; all values were determined on GC II agar base medium supplemented with 1% IsoVitaleX. These values are provided as a general guideline for the interpretation of disk-diffusion and Etest susceptibility results. The MICs and zone inhibition diameters may vary depending on many factors, including medium base, humidity, and age of the medium.
SPECIAL NOTES
At this time, the NCCLS has not published criteria for interpretation of susceptibilities of N. gonorrhoeae isolates to levofloxacin or azithromycin (1, 2, 9). Levofloxacin (250 mg, single dose, oral) is recommended by CDC for the treatment of uncomplicated gonorrhea in the United States (7). However, based on the pharmacokinetics of levofloxacin, it is estimated that gonococcal isolates with MICs ≥1.0 µg/ml of levofloxacin may be resistant to the CDC-recommended dose of this agent. It is recommended that, until CLSI establishes criteria for the interpretation of susceptibilities of gonococcal isolates to levofloxacin, that laboratories determine susceptibilities to either ciprofloxacin or ofloxacin and that isolates determined to be intermediate or resistant to either ciprofloxacin or ofloxacin be considered to exhibit corresponding levels of resistance to levofloxacin. Although CDC does not recommend azithromycin (2 g, single dose, oral) for the treatment of gonorrhea (8), this dose may be used to treat gonorrhea in areas of the United States where cefixime is no longer available and where fluoroquinolones can no longer be used because of the increase in prevalence of fluoroquinolone-resistance gonococcal strains. Currently, only limited data are available for assessing the clinical outcome of treatment of gonococcal infections with a 2g dose of azithromycin (10. More extensive assessments of treatment outcome associated with this regimen are required.
Until interpretive criteria for susceptibilities of gonococcal isolates to these agents are available, the NRL at CDC recommends that MICs ≥1.0 µg/ml of levofloxacin or azithromycin be considered critical MICs which require additional evaluation. When gonococcal infections have been treated with the CDC-recommended dose of levofloxacin (250 mg, single dose, oral) or with azithromycin (2 g, single dose, oral), the isolation of gonococcal isolates with critical MICs of ≥1.0 µg/ml of levofloxacin or azithromycin, respectively, from such infections should prompt further investigation to determine that such infections have been treated successfully.
It should be noted that the critical MIC of ≥1.0 µg/ml of azithromycin for the 2 g dose is not valid when evaluating susceptibilities of gonococcal isolates from infections treated with 1 g of azithromycin (which is not recommended by CDC for the treatment of uncomplicated gonorrhea but is recommended for the treatment of Chlamydia trachomatis infections). Evaluation of clinical treatment outcomes has indicated that gonococcal infections caused by strains with MICs of ≥0.125 µg/ml may fail to respond to treatment with the 1-g dose of azithromycin (11).
RECOMMENDED TESTING AND CONFIRMATORY TESTING
It is recommended that antimicrobial susceptibility testing be performed against agents that are being used as primary therapies against uncomplicated gonorrhea and against agents that would be used as alternative therapies should the primary agent(s) become ineffective for treating uncomplicated gonorrhea.
Healthcare providers and health departments can report suspected gonorrhea cephalosporin treatment failure or any N. gonorrhoeae specimen with decreased cephalosporin susceptibility through the Suspected Gonorrhea Treatment Failure Consultation Form.
CDC also recommends that isolates from certain infections be submitted to the CDC STD Lab for confirmation. These infections comprise those that fail to respond to CDC-recommended therapy and isolates determined to exhibit intermediate resistance or resistance.
Outside the United States, it is recommended that laboratories contact their local reference laboratory to ascertain criteria for interpreting antimicrobial susceptibilities and criteria for submitting isolates for confirmation of antimicrobial resistance.
BIBLIOGRAPHY
- NCCLS.* Performance standards for antimicrobial disk susceptibility tests; Approved standard – Eighth edition. Wayne, PA: NCCLS, 2003. NCCLS document no. M02-A8.
- NCCLS.* Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; Approved standard – Sixth edition. Wayne, PA: NCCLS, 2003. NCCLS document no. M07-A6.
- Whittington WL, Knapp JS. Trends in antimicrobial resistance in Neisseria gonorrhoeae in the United States. Sex Transm Dis 1988;15:202-210.
- Knapp JS, Fox KK, Trees DL, Whittington WL. Fluoroquinolone resistance in Neisseria gonorrhoeae. Emerg Inf Dis 1997;3:33-39.
- Fox KK, Knapp JS, Holmes KK, et al. Antimicrobial resistance in Neisseria gonorrhoeae in the United States, 1988-1994: the emergence of decreased susceptibility to the fluoroquinolones. J Infect Dis 1997;175:1396-1403.
- CDC. Sexually transmitted disease surveillance 2001 supplement: Gonococcal Isolate Surveillance Project (GISP) Annual Report – 2001. Atlanta, GA: U.S. Department of Health and Human Services; October 2001. Electronic reports beginning in 1998 are available from:
- CDC. Sexually Transmitted Diseases Treatment Guidelines 2002. MMWR 2002;51(No.RR-6). Available from:
- State and Local Health Alerts (retired URL: www./cdc.gov/std/gonorrhea/arg/health-alerts.htm).
- CLSI.* Performance standards for antimicrobial susceptibility testing; Fifteenth Informational Supplement. Wayne, PA: 2002. CLSI document no. M100-S15.
- Handsfield HH, Dalu ZA, Martin DH, Douglas JM Jr, McCarty ML, Schlossberg D. Multicenter trial of single-dose azithromycin vs. Ceftriaxone in the treatment of uncomplicated gonorrhea. Sex Transm Dis 1994;21:107-111.
- Young H, Moyes A, McMillan A. Azithromycin and erythromycin resistant Neisseria gonorrhoeae following treatment with azithromycin. Int J STD AIDS 1997;8:299-302.
- Wang SA, Lee MV, O’Connor N, Iverson CJ, Ohye RG, Whiticar PM, Hale JA, Trees DL, Knapp JS, Effler PV, Weinstock HS. Multidrug-resistant Neisseria gonorrhoeae with decreased susceptibility to cefixime-Hawaii, 2001. Clin Infect Dis. 2003; 15;37:849-52 Presentation available from: https://www.cdc.gov/iceid/webcast/antimicrobial1.htm
*Information on the most recent CLSI publications is available at: http://www.clsi.org/ (select “Shop” menu followed by “Microbiology” menu) or by contacting CLSI. CLSI, 940 West Valley Road, Suite 1400, Wayne, PA 19087-1898. Phone: 610 688 0100; fax: 610 688 0700; e-mail: exoffice@nccls.org . Note that performance standards are published each year.
Table 1. Critical zone inhibition diameters (mm) and MICs (µg/ml) for the interpretation of susceptibilities of strains of Neisseria gonorrhoeae to selected antimicrobial agents. (Criteria for all agents except levofloxacin and azithromycin are those recommended by CLSI; criteria for interpretation of susceptibilities are those recommended by the NRL.)
| Antimicrobial Agent |
Zone Inhibition Diameters (nearest whole mm) |
Equivalent MIC (µg/ml) |
||||
|---|---|---|---|---|---|---|
| R | I | S | R | I | S | |
| Penicillin (10 units) | ≤26 | 27-46 | ≥47 | ≥2.0 | 0.125-1.0 | ≤0.06 |
| Tetracycline (30 µg) | ≤30 | 31-37 | ≥38 | ≥2.0 | 0.5-1.0 | ≤0.25 |
| Spectinomycin (100 µg) | ≤14 | 15-17 | ≥18 | ≥128.0 | 64.0 | ≤32.0 |
| Ceftriaxone* (30 µg) | — | — | ≥35 | — | — | ≤0.25 |
| Cefixime* (5 µg) | — | — | ≥31 | — | — | ≤0.25 |
| Ciprofloxacin (5 µg) | ≤27 | 28-40 | ≥41** | ≥1.0 | 0.125-0.5 | ≤0.06 |
| Ofloxacin (5 µg) | ≤24 | 25-30 | ≥31 | ≥2.0 | 0.5-1.0 | ≤0.25 |
| Levofloxacin (5 µg) | . | . | . | ≥1.0 *** | . | . |
| Azithromycin (15 µg) | ≤30 *** | . | . | ≥1.0 *** | . | . |
Abbreviations: MIC, minimal inhibitory concentration; R, resistant; I, intermediate; S, susceptible.
*Criteria for intermediate and resistant not available; strains have not been confirmed to cause clinical treatment failure. However, four isolates with MICs exhibiting decreased susceptibility to cefixime (MIC, range 0.25 – 1.0 µg/ml; modal MIC, 0.5 µg/ml) were identified among gonococcal isolates from Hawaii in 2001 (12).
**Preliminary MIC data suggest that isolates exhibiting zone inhibition diameters of ciprofloxacin of 35 to 41 mm are susceptible to ciprofloxacin (MIC ≤0.06 µg/ml). It is recommended that susceptibilities of isolates exhibiting zone inhibition diameters of 35 to 41 mm be confirmed by agar dilution susceptibility testing before isolates are categorized as being intermediate to ciprofloxacin.
***In the absence of CLSI-established criteria, the NRL recommends the use of critical MICs ≥1.0 µg/ml to interpret susceptibilities of gonococcal isolates to these agents until more extensive assessments of clinical treatment outcome to these agents are available (see Special Notes). When gonococcal infections have been treated with the CDC-recommended dose of levofloxacin (250 mg, single dose, oral) or with azithromycin (2 g, single dose, oral), the isolation of gonococcal isolates with critical MICs of ≥1.0 µg/ml of levofloxacin or azithromycin, respectively, from such infections should prompt further investigation to determine that such infections have been treated successfully. The NRL would appreciate receiving isolates with MICs of ≥1.0 µg/ml of levofloxacin or azithromycin, or zone inhibition diameters of ≤30 mm of azithromycin for confirmation of susceptibilities.
Table 2a. Ranges of zone inhibition diameters (mm)* and equivalent MIC (µg/ml) range for reference strains of Neisseria gonorrhoeae
| Antimicrobial Agent |
Strain | Range of MICs and corresponding disk diffusion inhibition zone diameters (mm) | |||
|---|---|---|---|---|---|
| ATCC 49226a | F-28 | P681E | CDC 10328 | ||
| Phenotypeb | Susceptible | SpcR | PP-TR | CipI | |
| Penicillin (10 units) | MIC Range Disk |
0.25-1.0 [26-34] |
0.015-0.06 [37-47] |
2.0-≥64.0 [10-20] |
4.0-≥32.0 [ND] |
| Tetracycline (30 µg) | MIC Range Disk |
0.25-1.0 [30-42] |
0.125-0.5 [35-40] |
8.0-32.0 [14-19] |
0.25-1.0 [ND] |
| Spectinomycin (100 µg) | MIC Range Disk |
8.0-32.0 [23-29] |
>128.0 [6-7] |
[≤128.0] [22-25] |
[≤128.0] [ND] |
| Ceftriaxone* (30 µg) | MIC Range Disk |
0.004-0.015 [39-51] |
0.0005-0.002 [49-62] |
0.002-0.008 [43-53] |
0.002-0.015 [ND] |
| Cefixime* (5 µg) | MIC Range Disk |
0.004-0.03 [37-45] |
0.001-0.008 [ND] |
0.008-0.03 [ND] |
0.004-0.03 [ND] |
| Ciprofloxacin (5 µg) | MIC Range Disk |
0.001-0.008 [48-58] |
0.001-0.004 [40-55] |
0.002-0.008 [45-55] |
0.125-0.5 [30-34] |
| Ofloxacin (5 µg) | MIC Range Disk |
0.004-0.015 [43-51] |
0.004-0.015 [40-55] |
0.004-0.015 [40-50] |
0.25-1.0 [27-32] |
| Azithromycin (15 µg) | MIC Range Disk |
0.125-0.5 [27-36]d |
0.03-0.125 [ND] |
0.03-0.125 [ND] |
0.015-0.125 [ND] |
| ß-lactamase | . | – | – | + | + |
| Antimicrobial Agent |
Strain | Range of MICs and corresponding disk diffusion inhibition zone diameters (mm) | ||
|---|---|---|---|---|
| CDC 10329 | SPJ-15 | SPL-4 | ||
| Phenotypeb | CipR | AznCc | CfxDS | |
| Penicillin (10 units) | MIC Range Disk |
16.0-≥64.0 [ND] |
0.5-1.0 [ND] |
4.0-16.0 [ND] |
| Tetracycline (30 µg) | MIC Range Disk |
2.0-4.0 [ND] |
1.0-4.0 [ND] |
2.0-8.0 [ND] |
| Spectinomycin (100 µg) | MIC Range Disk |
[≤128.0] [ND] |
[≤128.0] [ND] |
[≤128.0] [ND] |
| Ceftriaxone* (30 µg) | MIC Range Disk |
0.004-0.03 [ND] |
0.004-0.015 [ND] |
0.03-0.25 [ND] |
| Cefixime* (5 µg) | MIC Range Disk |
0.008-0.06 [ND] |
0.008-0.06 [ND] |
0.25-0.5 [ND] |
| Ciprofloxacin (5 µg) | MIC Range Disk |
1.0-2.0 [21-26] |
0.004-0.008 [ND] |
8.0-32.0 [ND] |
| Ofloxacin (5 µg) | MIC Range Disk |
2.0-4.0 [18-21] |
0.008-0.03 [ND] |
16.0 [ND] |
| Azithromycin (15 µg) | MIC Range Disk |
0.125-0.5 [ND] |
1.0-8.0 [19-23]d |
0.125-0.5 [ND] |
| ß-lactamase | . | + | – | – |
Abbreviations: MIC, minimal inhibitory concentration (µg/ml) ; SpcR, spectinomycin resistant (MIC >128.0 µg/ml); PP-TR, penicillinase-producing with TetMconjugative plasmid; CipI, intermediate to ciprofloxacin (MIC 0.125-0.5 µg/ml); CipR, ciprofloxacin-resistant (MIC ≥1.0 µg/ml); AznC, isolates exhibiting MICs of ≥1.0 µg/ml of azithromycin (see Special Notes in text); CfxDS, decreased susceptibility to cefixime (MIC >0.25 µg/ml of cefixime); [ND], not determined; +, ß-lactamase positive; -, ß-lactamase negative.
a ATCC 49226 is the NCCLS-recommended quality control strain. MICs and inhibition zone diameters for ATCC 49226 are those recommended by the NCCLS except for MICs for azithromycin which were derived from interlaboratory testing by six laboratories.
b Phenotypes refer only to the susceptibilities for which the quality control strain is intended; strains may also exhibit additional resistance(s) of interest.
c SPJ-15, isolate with a critical MIC ≥1.0 µg/ml of azithromycin (see Special Notes ).
d Zone inhibition diameters have not been determined for cefixime at this time and ranges for azithromycin may be extended as more measurements are made.
Table 2b. Quality control values for antimicrobial susceptibilities of reference strains of Neisseria gonorrhoeae determined by agar dilution or Etest
| Antimicrobial Agent |
Strain | MIC (µg/ml) | |||
|---|---|---|---|---|---|
| ATCC 49226a | F-28 | P681E | CDC 10328 | ||
| Phenotypeb | Susceptible | SpcR | PP-TR | CipI | |
| Penicillin | MIC Range Modal MIC |
0.25-1.0 [1.0] |
0.015-0.06 [0.03] |
2.0-≥64.0 [8.0] |
4.0-≥32.0 [ND] |
| Tetracycline | MIC Range Modal MIC |
0.25-1.0 [1.0] |
0.125-0.5 [0.25] |
8.0-32.0 [16.0] |
0.25-1.0 [0.5-1.0] |
| Spectinomycin | MIC Range Modal MIC |
8.0-32.0 [ND] |
>128.0 [>128.0] |
[≤128.0] [≤128.0] |
[≤128.0] [≤128.0] |
| Ceftriaxone* | MIC Range Modal MIC |
0.004-0.015 [0.015] |
0.0005-0.002 [0.001] |
0.002-0.008 [0.004] |
0.002-0.015 [0.004] |
| Cefixime* | MIC Range Modal MIC |
0.004-0.03 [0.03] |
0.001-0.008 [0.002] |
0.008-0.03 [0.015] |
0.004-0.03 [0.008-0.015] |
| Ciprofloxacin d | MIC Range Modal MIC |
0.001-0.008 [0.004] |
0.001-0.004 [0.002] |
0.002-0.008 [0.004] |
0.125-0.5 [0.25] |
| Ofloxacind | MIC Range Modal MIC |
0.004-0.015 [0.004] |
0.004-0.015 [0.008] |
0.004-0.015 [0.008] |
0.25-1.0 [0.5] |
| Levofloxacind | MIC Range Modal MIC |
0.008 [0.008] |
0.004 [0.004] |
0.004 [0.004] |
0.25-0.5 [0.25-0.5] |
| Azithromycin | MIC Range Modal MIC |
0.125-0.5 [0.25-0.5] |
0.03-0.125 [0.06] |
0.03-0.125 [0.06] |
0.015-0.125 [0.03] |
| ß-lactamase | . | – | – | + | + |
| Antimicrobial Agent |
Strain | MIC (µg/ml) | ||
|---|---|---|---|---|
| CDC 10329 | SPJ-15 | SPL-4 | ||
| Phenotypeb | CipR | AznCc | CfxDS | |
| Penicillin | MIC Range Modal MIC |
16.0-≥64.0 [ND] |
0.5-1.0 [0.5] |
4.0-16.0 [8.0] |
| Tetracycline | MIC Range Modal MIC |
2.0-4.0 [2.0-4.0] |
1.0-4.0 [1.0-2.0] |
2.0-8.0 [4.0] |
| Spectinomycin | MIC Range Modal MIC |
[≤128.0] [≤128.0] |
[≤128.0] [≤128.0] |
[≤128.0] [≤128.0] |
| Ceftriaxone* | MIC Range Modal MIC |
0.004-0.03 [0.008-0.015] |
0.004-0.015 [0.008] |
0.03-0.25 [0.125] |
| Cefixime* | MIC Range Modal MIC |
0.008-0.06 [0.015] |
0.008-0.06 [0.03] |
0.25-0.5 [0.5] |
| Ciprofloxacind | MIC Range Modal MIC |
1.0-2.0 [1.0] |
0.004-0.008 [0.004] |
8.0-32.0 [8.0-16.0] |
| Ofloxacind | MIC Range Modal MIC |
2.0-4.0 [2.0] |
0.008-0.03 [0.015] |
16.0 [16.0] |
| Levofloxacind | MIC Range Modal MIC |
1.0-4.0 [2.0] |
0.008-0.015 [0.008-0.015] |
8.0 [8.0] |
| Azithromycin | MIC Range Modal MIC |
0.125-0.5 [0.25-0.5] |
1.0-8.0 [2.0-4.0]d |
0.125-0.5 [0.25] |
| ß-lactamase | . | + | – | – |
Abbreviations: MIC, minimal inhibitory concentration (µg/ml) ; SpcR, spectinomycin resistant (MIC >128.0 µg/ml); PP-TR, penicillinase-producing with TetMconjugative plasmid; CipI, intermediate to ciprofloxacin (MIC 0.125-0.5 µg/ml); CipR, resistant to ciprofloxacin (MIC ≥1.0 µg/ml); AznC, isolates with MICs of 1.0 µg/ml of azithromycin (see Footnote c); CfxDS, decreased susceptibility to cefixime (MIC >0.25 µg/ml of cefixime); [ND], not determined; +, ß-lactamase positive; -, ß-lactamase negative.
a ATCC 49226 is the NCCLS-recommended quality control strain. MICs for ATCC 49226 are those recommended by the NCCLS; MICs of azithromycin were determined by interlaboratory testing by six laboratories in the United States.
b Phenotypes refer only to the susceptibilities for which the quality control strain is intended; strains may also exhibit additional resistance(s) of interest.
c SPJ-15, isolate with a critical MIC ≥1.0 µg/ml of azithromycin (see Special Notes ).
d MICs of levofloxacin are based on a limited number of tests.
In the absence of NCCLS-recommended criteria for the interpretation of susceptibilities of levofloxacin, it is recommended that laboratories determine susceptibilities to either ciprofloxacin or ofloxacin as an indication of resistance to levofloxacin.
Preparation of McFarland Turbidity Standard
An opacity standard is prepared by mixing barium chloride (BaCl2) and sulfuric acid (H2SO4). The resultant precipitate, barium sulfate (BaSO4), is very insoluble. A new standard should be prepared every six months.
- Prepare the turbidity standard by adding 0.5 ml of 0.048 M BaCl2 (1.175% w/v BaCl2.2H2O) to 99.5 ml of 0.36 N H2SO4 (1% v/v)
- Distribute 4 to 6 ml into screw-cap tubes of the same size as those used in preparing the culture suspension.
- Tightly seal these tubes and store them in the dark at room temperature.
- Shake the turbidity standard vigorously on a mechanical vortex mixer just before use.