At a glance
CDC's Newborn Screening Laboratories help assure the early and accurate detection of treatable newborn diseases.
More Information
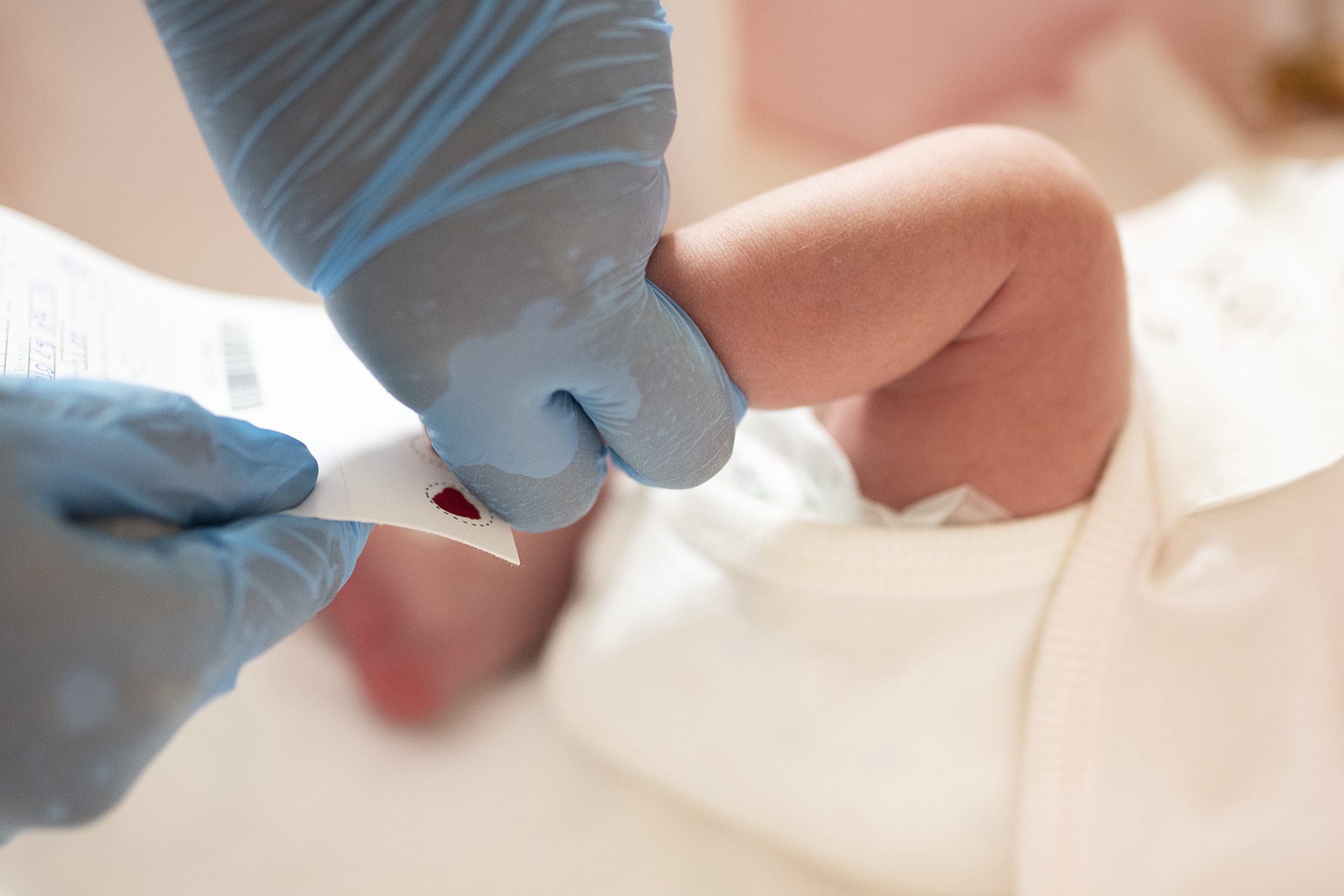
Newborn Screening Quality Assurance Program
The Newborn Screening Quality Assurance Program (NSQAP) develops analytical methods to measure substances in dried blood spots (DBSs) and produces certified DBS quality-control and reference materials for newborn screening tests. Because of NSQAP, parents and doctors in the United States and worldwide can trust the results of newborn screening tests.
Newborn Screening Quality Assurance Program | Laboratory Quality Assurance Programs | CDC
To access previous newborn quality assurance reports: Program Reports | Newborn Screening | CDC
Access the NSQAP Participant Portal
A2LA Accredited Proficiency Testing Provider
Supporting state newborn screening laboratories
CDC enhances nation-wide capacity and capability to conduct newborn screening by providing resources and technical assistance to state public health laboratories.
Supporting State Newborn Screening Laboratories | Newborn Screening | CDC
Emergency preparedness for newborn screening programs
CDC helps state newborn screening programs be prepared for emergencies that could disrupt normal operations.
Emergency Preparedness for Newborn Screening Programs | Newborn Screening | CDC
Enhancing data-driven disease detection in Newborns
The Enhancing Data-driven Disease Detection in Newborns (ED3N) pilot program aims to provide a national data platform that will improve the quality and interpretation of newborn screening results.
Enhancing Data-Driven Disease Detection in Newborns | Newborn Screening | CDC
Contacts
Please note that CDC cannot respond to questions about individual medical cases, provide second opinions, or make specific recommendations regarding therapy. Those issues should be addressed directly with your health care provider.
E-mail: CDC-INFO
Phone: CDC Health Line 1-800-CDC-INFO (1-800-232-4636)
Address:
Division of Laboratory Sciences
Mail Stop F-204770 Buford Highway
NE Atlanta, GA 30341-3724
