Notes from the Field: Circulating Vaccine-Derived Poliovirus Type 2 Emergences Linked to Novel Oral Poliovirus Vaccine Type 2 Use — Six African Countries, 2021–2023
Weekly / September 22, 2023 / 72(38);1041–1042
Elizabeth Davlantes, MD1; Jaume Jorba, PhD2; Elizabeth Henderson2; Kelley Bullard, MS2; Mark. A. Deka, PhD3; Anfumbom Kfutwah, PhD4; Javier Martin, PhD5; Maël Bessaud, PhD6; Lester M. Shulman, PhD7; Kaija Hawes, MBA8; Ousmane M. Diop, PhD9; Ananda S. Bandyopadhyay, MBBS8; Simona Zipursky, MSc9; Cara C. Burns, PhD2 (View author affiliations)
View suggested citationAltmetric:
Circulating vaccine-derived poliovirus (cVDPV) outbreaks can occur when oral poliovirus vaccine strains (most often, Sabin monovalent oral poliovirus vaccine type 2 [mOPV2]) undergo prolonged circulation in undervaccinated populations, resulting in genetic reversion to neurovirulence. A novel type 2 oral poliovirus vaccine (nOPV2) has been developed, which has been shown in clinical trials to be less likely than mOPV2 to revert to paralytic variants and to have limited genetic modifications in initial field use (1–4). Approximately 700 million doses of nOPV2 have been administered worldwide in response to outbreaks of cVDPV type 2 (cVDPV2). cVDPV2 detections originating from nOPV2 use from initial rollout during March 2021–September 7, 2023, are described in this report.
Investigation and Outcomes
Polio surveillance and laboratory data collected through the Global Polio Eradication Initiative were reviewed. During August 2021–July 2023, seven cVDPV2 emergences of nOPV2 origin from 61 paralytic cases and 39 environmental surveillance (sewage) samples were detected in six countries, all in Africa: Burundi, Central African Republic (CAR), Democratic Republic of the Congo (DRC), Nigeria, Tanzania, and Zambia (Figure). The isolates have limited divergence from the parental nOPV2 vaccine strain in the VP1 capsid protein coding area (six to 16 nucleotide substitutions), indicating that surveillance detected emergence relatively early after vaccination. This activity was reviewed by CDC, deemed not research, and was conducted consistent with applicable federal law and CDC policy.*
Circulation of four of the seven cVDPV2 emergences derived from nOPV2 use has been detected only in subnational areas of the countries in which they originated (CAR, DRC, and Nigeria), two have spread to other areas of the originating country (CAR and DRC), and one has spread from the originating country (DRC) to neighboring countries (Burundi, Tanzania, and Zambia). Three emergences spreading within or outside of the originating country (all from DRC) might be ongoing, with most recent detections in July 2023. The largest and widest-spreading emergence, RDC-SKV-1, was first detected in DRC’s South Kivu province in September 2022; viruses from this emergence have been identified in six provinces of DRC and in neighboring Burundi, Tanzania, and Zambia. RDC-SKV-1 has been detected as recently as July 2023 in Tanzania.
Preliminary Conclusions and Actions
The potential for mutation and reversion to neurovirulence is a rare but recognized risk for all live attenuated oral poliovirus vaccines; extensive use of nOPV2 worldwide since March 2021 suggests that reversion occurs less frequently than with mOPV2 (4). A preliminary estimate suggests that cVDPV2 emergences occur after mOPV2 use at a rate of one emergence per 10 million mOPV2 doses administered; for nOPV2, this rate is approximately 10 times lower, at one emergence per 100 million doses. As with all cVDPV emergences, cVDPV2 outbreaks of nOPV2 origin are more likely to occur when nOPV2 supplementary immunization activities (SIAs) do not achieve high coverage in populations with persistently low immunity against polioviruses (5). To combat all cVDPV2 outbreaks and reduce future emergences, responding with prompt SIAs that reach all targeted children is essential.
Acknowledgments
National Institute for Communicable Diseases of South Africa; Institut Pasteur of Bangui, Central African Republic; Insitut Pasteur, France; National Institute of Biomedical Research, Democratic Republic of the Congo; Feyrouz Kurji.
Corresponding author: Elizabeth Davlantes, Lyo2@cdc.gov.
1Global Immunization Division, Global Health Center, CDC; 2Division of Viral Diseases, National Center for Immunization and Respiratory Diseases, CDC; 3Geospatial Research, Analysis, and Services Program, Agency for Toxic Substances and Disease Registry, Atlanta, Georgia; 4Regional Polio Laboratory Network, World Health Organization Regional Office for Africa, Brazzaville, Republic of the Congo; 5Division of Vaccines, Medicines and Healthcare products Regulatory Agency, Potters Bar, United Kingdom; 6Institut Pasteur, Université Paris Cité, Virus Sensing and Signaling Unit, Paris, France; 7Central Virology Laboratory, Public Health Services, Israel Ministry of Health, Tel Aviv, Israel; 8Bill & Melinda Gates Foundation, Polio Team, Seattle, Washington; 9World Health Organization, Geneva, Switzerland.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. Javier Martin reports institutional support from the Bill & Melinda Gates Foundation. Lester M. Shulman reports support from McKing Consulting Corporation for technical consultation on nOPV2, grant support from the World Health Organization, travel support from the Bill & Melinda Gates Foundation to attend the February 2023 nOPV2 work group meeting in London, and voluntary position as recording secretary for the Israel National Polio Certification Committee and for the Israel Emergency Response Team during 2022–2023. No other potential conflicts of interest were disclosed.
* 45 C.F.R. part 46.102(l)(2), 21 C.F.R. part 56; 42 U.S.C. Sect. 241(d); 5 U.S.C. Sect. 552a; 44 U.S.C. Sect. 3501 et seq.
References
- Yeh MT, Bujaki E, Dolan PT, et al. Engineering the live-attenuated polio vaccine to prevent reversion to virulence. Cell Host Microbe 2020;27:736–751.e8. https://doi.org/10.1016/j.chom.2020.04.003 PMID:32330425
- Martin J, Burns CC, Jorba J, et al. Genetic characterization of novel oral polio vaccine type 2 viruses during initial use phase under Emergency Use Listing—worldwide, March–October 2021. MMWR Morb Mortal Wkly Rep 2022;71:786–90. https://doi.org/10.15585/mmwr.mm7124a2 PMID:35709073
- Zaman K, Bandyopadhyay AS, Hoque M, et al. Evaluation of the safety, immunogenicity, and faecal shedding of novel oral polio vaccine type 2 in healthy newborn infants in Bangladesh: a randomised, controlled, phase 2 clinical trial. Lancet 2023;401:131–9. https://doi.org/10.1016/S0140-6736(22)02397-2 PMID:36495882
- Bandyopadhyay AS, Zipursky S. A novel tool to eradicate an ancient scourge: the novel oral polio vaccine type 2 story. Lancet Infect Dis 2023;23:e67–71. https://doi.org/10.1016/S1473-3099(22)00582-5 PMID:36162417
- Pons-Salort M, Burns CC, Lyons H, et al. Preventing vaccine-derived poliovirus emergence during the polio endgame. PLoS Pathog 2016;12:e1005728. https://doi.org/10.1371/journal.ppat.1005728 PMID:27384947
 FIGURE. Detections of circulating vaccine-derived poliovirus type 2 linked to novel oral poliovirus type 2 vaccine use, by emergence group — Africa, 2021–2023
FIGURE. Detections of circulating vaccine-derived poliovirus type 2 linked to novel oral poliovirus type 2 vaccine use, by emergence group — Africa, 2021–2023
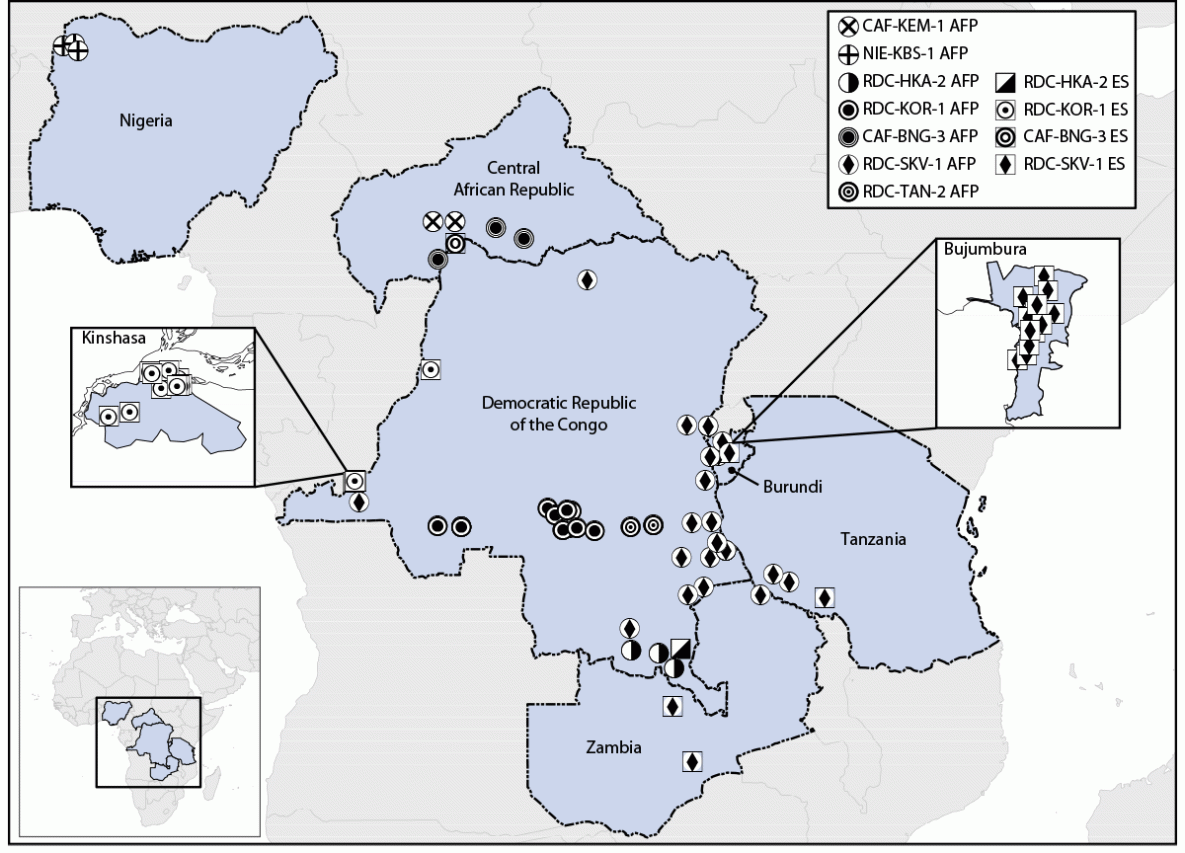
Abbreviations: AFP = acute flaccid paralysis case; ES = environmental surveillance isolate.
Suggested citation for this article: Davlantes E, Jorba J, Henderson E, et al. Notes from the Field: Circulating Vaccine-Derived Poliovirus Type 2 Emergences Linked to Novel Oral Poliovirus Vaccine Type 2 Use — Six African Countries, 2021–2023. MMWR Morb Mortal Wkly Rep 2023;72:1041–1042. DOI: http://dx.doi.org/10.15585/mmwr.mm7238a4.
MMWR and Morbidity and Mortality Weekly Report are service marks of the U.S. Department of Health and Human Services.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All HTML versions of MMWR articles are generated from final proofs through an automated process. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (https://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.