Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail.
Malaria Surveillance --- United States, 2008
Please note: An erratum has been published for this article. To view the erratum, please click here.
Abstract
Problem/Condition: Malaria in humans is caused by intraerythrocytic protozoa of the genus Plasmodium. These parasites are transmitted by the bite of an infective female Anopheles species mosquito. The majority of malaria infections in the United States occur among persons who have traveled to areas with ongoing malaria transmission. In the United States, cases can occur through exposure to infected blood products, congenital transmission, or local mosquitoborne transmission. Malaria surveillance is conducted to identify episodes of local transmission and to guide prevention recommendations for travelers.
Period Covered: This report summarizes cases in patients with onset of illness in 2008 and summarizes trends during previous years.
Description of System: Malaria cases diagnosed by blood film, polymerase chain reaction, or rapid diagnostic tests are mandated to be reported to local and state health departments by health-care providers or laboratory staff members. Case investigations are conducted by local and state health departments, and reports are transmitted to CDC through the National Malaria Surveillance System (NMSS), National Notifiable Diseases Surveillance System (NNDSS), and direct CDC consultations. Data from these reporting systems are the basis for this report.
Results: CDC received reports of 1,298 cases of malaria with an onset of symptoms in 2008 among patients in the United States, a decrease of 13.8% from the 1,505 cases reported for 2007 (p<0.001). These cases included one transfusion-related case, one congenital case, and two fatal cases. Plasmodium falciparum, P. vivax, P. malariae, and P. ovale were identified in 40.6%, 14.6%, 1.5%, and 1.4% of cases, respectively. The first documented case of simian malaria, P. knowlesi, was reported in a U.S. traveler. Eight (0.6%) of the 1,298 patients were infected by two or more species. The infecting species was unreported or undetermined in 41.2% of cases. Based on estimated volume of travel from the World Tourism Organization, the highest estimated relative case rates of malaria among travelers occurred among those returning from countries in West Africa. A total of 508 U.S. civilians acquired malaria abroad; among the 480 civilians for whom chemoprophylaxis information was known, 344 (71.7%) reported that they had not followed a chemoprophylactic drug regimen recommended by CDC for the area to which they had traveled. Fourteen cases were reported in pregnant women, among whom none adhered to a complete prevention drug regimen.
Interpretation: A significant decrease in the number of malaria cases occurred from 2007 to 2008. No change occurred in the proportions of cases caused by the various Plasmodium species. U.S. civilians traveling to countries in West Africa had the highest estimated relative case rates. In the majority of reported cases, U.S. civilians who acquired malaria abroad had not adhered to a chemoprophylaxis regimen that was appropriate for the country in which they acquired the infection.
Public Health Actions: Persons traveling to an area in which malaria is endemic should take steps to prevent malaria, which might include taking one of the recommended chemoprophylaxis regimens appropriate for the region of travel and using personal protection measures to prevent mosquito bites. Any person who has been to a malarious area and who subsequently develops a fever or influenza-like symptoms should seek medical care immediately and report their travel history to the clinician; investigation should always include blood-film tests for malaria with results available immediately. Malaria infections can be fatal if not diagnosed and treated promptly. Malaria prevention recommendations are available from CDC online (http://wwwn.cdc.gov/travel/contentDiseases.aspx#malaria) or by calling the Malaria Hotline (telephone 770-488-7788). Malaria treatment recommendations can be obtained from CDC online (http://www.cdc.gov/malaria/diagnosis_treatment/treatment.htm) or by calling the Malaria Hotline.
Introduction
Malaria in humans is caused by infection with one or more species of Plasmodium parasite (i.e., P. falciparum, P. vivax, P. ovale, P. malariae, and occasionally other Plasmodium species). The infection is transmitted by the bite of an infective female Anopheles species mosquito. P. falciparum and P. vivax species cause the most infections worldwide. P. falciparum is the species that most commonly causes severe and potentially fatal malaria. Worldwide, an estimated 243 million clinical cases and 863,000 deaths occurred in 2008, primarily among children aged <5 years living in sub-Saharan Africa (1). P. vivax and P. ovale have dormant liver-stage parasites, which can reactivate and cause malaria several months or years after the infecting mosquito bite. P. malariae can result in long-lasting infections and if untreated can persist asymptomatically in the human host for years, even a lifetime (1). P. knowlesi, a parasite of Asian macaques, has been documented as a cause of human infections, including some deaths, in Southeast Asia. Investigations are ongoing to determine the extent of P. knowlesi transmission to humans (2). Forty-nine percent of the world's population lives in areas where malaria is transmitted (i.e., 109 countries in parts of Africa, Asia, the Middle East, Eastern Europe, Central America and South America, the Caribbean, and Oceania). Before the 1950s, malaria was endemic throughout the southeastern United States; an estimated 600,000 cases occurred in 1914 (3). During the late 1940s, a combination of improved housing and socioeconomic conditions, environmental management, vector-control efforts, and case management interrupted malaria transmission in the United States. Since then, malaria case surveillance has been maintained to detect locally acquired cases that could indicate the reintroduction of transmission and to monitor patterns of resistance to antimalarial drugs. Anopheles mosquitoes remain seasonally present in all states and territories except Hawaii.
The majority of reported malaria cases diagnosed each year in the United States are imported from regions where malaria transmission is known to occur, although congenital infections and infections resulting from exposure to blood or blood products also are reported in the United States. In addition, a limited number of cases are occasionally reported that might have been acquired through local mosquitoborne transmission (4).
Although the signs and symptoms of malaria illness vary, the majority of patients have fever; other common signs and symptoms include headache, back pain, chills, increased sweating, myalgia, nausea, vomiting, diarrhea, and cough. The diagnosis of malaria should always be considered for persons with these symptoms who have traveled to an area with known malaria transmission. Malaria also should be considered in the differential diagnosis of persons who have fever of unknown origin, regardless of their travel history. Untreated infections can rapidly progress to coma, renal failure, acute respiratory distress syndrome (ARDS), and death.
Malaria is a nationally notifiable disease; clinicians and health-care facilities are mandated by legislation or state and local regulations to report cases to their state health department (5). State and local health departments and CDC investigate malaria cases acquired in the United States, and CDC analyzes data from imported cases to detect trends in acquisition. This information is used to guide malaria prevention recommendations for international travelers. This report summarizes malaria cases reported to CDC among patients with onset of symptoms in 2008.
Methods
Data Sources
Malaria case data are reported to the National Malaria Surveillance System (NMSS) and the National Notifiable Diseases Surveillance System (NNDSS) (5). Although both systems rely on passive reporting, the numbers of reported cases might differ because of differences in collection and transmission of data. A substantial difference between the two systems is that NMSS receives more detailed clinical and epidemiologic data than NNDSS regarding each case (e.g., information concerning the area to which the infected person has traveled). Malaria cases can be reported to CDC through NMSS, NNDSS, or direct consultation with CDC; case data collected through these various paths are compared and compiled, duplicates are eliminated, and aggregate data are analyzed. This report presents results of the analysis of cases reported to CDC through all reporting systems.
Health-care providers or laboratories identify malaria cases among civilians and military personnel by blood film, polymerase chain reaction (PCR), or rapid diagnostic test (RDT).* Each confirmed malaria case is reported to local or state health departments and to CDC. CDC staff members review all reports when received and request additional information from the reporting health-care provider or health department if necessary (e.g., when no recent travel to a malarious country is reported). Some cases are reported by telephone directly to CDC by health-care providers, usually when they are seeking assistance with diagnosis or treatment. Information regarding cases reported directly to CDC is shared with the relevant state health department. All cases that are reported as having been acquired in the United States are investigated further, including all induced and congenital cases and possible introduced or cryptic cases. Information derived from uniform case report forms is entered into a database and analyzed annually.
An estimated case rate for each country was calculated using estimates of travel volume for U.S. travelers to each country where cases of malaria were acquired and the number of cases among U.S. travelers attributable to each country. Data used to estimate country-specific relative case rates were extrapolated from World Tourism Organization estimates of annual numbers of U.S. travelers to specified countries (6). Estimated relative case rates were determined by dividing the individual country-specific case rates by the median individual country-specific case rate.
The chi-square test was used to calculate p values and assess differences between variables reported in 2008 compared with previous years. A p value of <0.05 was considered statistically significant.
Definitions
The following definitions are used in this report:
- U.S. residents: Persons living in the United States; includes both civilians and U.S. military personnel, regardless of legal citizenship.
- U.S. civilians: U.S. residents, excluding U.S. military personnel.
- Foreign residents: Persons who have resident status in a country other than the United States.
- Visiting friends and relatives (VFR) travelers: Immigrants who are ethnically and racially distinct from the major population of their country of residence (a country in which malaria is not endemic) who return to their country of origin (where malaria is endemic) to visit friends or relatives. Included in the VFR category are family members such as a spouse or children who were born in the country of residence (2).
- Laboratory criteria for diagnosis: Demonstration of malaria parasites on blood film or by PCR or RDT.
- Confirmed case (7): Symptomatic or asymptomatic infection that occurs in a person in the United States or one of its territories who has malaria parasitemia that is laboratory confirmed (by microscopy or by PCR or RDT that is subsequently confirmed by microscopy), regardless of whether the person has had previous episodes of malaria while in other countries. A subsequent episode of malaria is counted as an additional case if the indicated Plasmodium species differs from the initially identified species. A subsequent episode of malaria that occurs in a person while in the United States could indicate a relapsing infection or treatment failure resulting from drug resistance if the indicated Plasmodium species is the same species identified previously. This report also uses terminology derived from the recommendations of the World Health Organization (WHO) (8). Definitions of the following terms are included for reference:
- Autochthonous malaria:
- Indigenous. Mosquitoborne transmission of malaria in a geographic area where malaria occurs regularly.
- Introduced. Mosquitoborne transmission of malaria from a person with an imported case in an area where malaria does not occur regularly.
- Imported malaria: Malaria acquired outside a specific area. In this report, imported cases are those acquired outside the United States and its territories.
- Induced malaria: Malaria acquired through artificial means (e.g., blood transfusion, organ transplantation, or by using shared syringes).
- Relapsing malaria: Recurrence of disease after it has been apparently cured. In malaria, true relapses are caused by reactivation of dormant liver-stage parasites (hypnozoites) found in P. vivax and P. ovale.
- Cryptic malaria: A case of malaria for which epidemiologic investigations fail to identify a plausible mode of acquisition. (This term applies primarily to cases identified in countries where malaria is not endemic.)
Laboratory Diagnosis of Malaria
To diagnose malaria early and promptly, physicians must obtain a travel history from every febrile patient. Malaria should be included in the differential diagnosis of every febrile patient who has traveled to a malarious area. If malaria is suspected, a Giemsa-stained film of peripheral blood should be examined for parasites as soon as possible. Thick and thin blood films must be prepared correctly because diagnostic accuracy depends on blood-film quality and examination by experienced laboratory personnel (9). Some reference laboratories and health departments can diagnose malaria using PCR, although this method is generally reserved for cases for which blood-film diagnosis of malaria is inadequate and for confirmation of species. PCR results also are often not available quickly enough to be useful for the initial diagnosis of a patient with malaria.
In addition, BinaxNOW Malaria, an RDT that detects circulating malaria-specific antigens, is widely available for use by U.S. laboratories. The test is only approved for use by hospital and commercial laboratories, not by individual clinicians or the general public (9). In the United States, use of the RDT can decrease the time required to make a malaria diagnosis but does not eliminate the need for the standard tests (10). Positive and negative RDTs must be confirmed by microscopy. RDTs are not able to determine the species or density of malaria parasites (11).
Results
General Surveillance
In 2008, CDC received 1,298 reports concerning cases of malaria among persons in the United States and its territories, representing a 13.8% decrease (p<0.001) from the 1,505 cases reported with onset in 2007 (9). In 2008, a total of 529 cases occurred among U.S. residents, 176 cases among foreign residents, and 593 cases among patients with unknown resident status (Table 1). Among cases in patients with known resident status, no significant change occurred between 2007 and 2008 in the proportion of cases among U.S. residents and foreign residents (p = 0.417). However, the proportion of cases among patients with unknown resident status changed significantly between 2007 and 2008 (p<0.001), with an increase of almost 17%.
Total malaria cases are compiled from two surveillance system: NNDSS and NMSS. Although most of these cases are reported to both systems, in 2007 and 2008, a total of 273 and 445 more cases were reported to NNDSS than NMSS, respectively, an increase of 63% from 2007 to 2008 (p<0.001). Although more cases appear to have been reported to NNDSS during 2007--2008, species type, resident status, prophylaxis use, and treatment regimens are not known for approximately 34% of the cases with onset in 2008. Of the cases reported via NMSS, the proportion of completed case forms with accompanying epidemiologic and clinical information in 2008 seem to be consistent with 2007, although 5% fewer cases included species type (p = 0.013).
Plasmodium Species
Among the 1,298 cases reported in 2008, the infecting Plasmodium species was identified and reported in 764 (58.9%) cases (Table 2). P. falciparum and P. vivax comprised the majority of infections and were identified in 69.0% and 25.0% of infected patients with species reported, respectively. In addition, one confirmed case of P. knowlesi was reported. Among 719 cases for which both the region of acquisition and the infecting species were known, P. falciparum accounted for 90.9% of infections acquired in Africa, 50.0% in the Americas, 11.5% in Asia, and 10.0% in Oceania. Infections attributed to P. vivax accounted for 3.0% acquired in Africa, 47.7% in the Americas, 82.2% in Asia, and 90.0% in Oceania.
Region of Acquisition and Diagnosis
All but two cases were reported as imported cases (one congenital case and one cryptic case, which were not included in the analysis of imported cases). Among 792 imported cases for which the region of acquisition was known, 563 (71.1%) were acquired in Africa, 170 (21.5%) in Asia, 49 (6.2%) in the Americas, and 10 (1.3%) in Oceania (Table 3). Countries in West Africa accounted for 489 (86.8%) cases acquired in Africa, and India accounted for 114 (67.1%) cases acquired in Asia. In the Americas, a total of 40 (81.6%) cases were acquired in Central America and the Caribbean, of which 35 (87.5%) cases were from Haiti and Honduras combined. Six (12.2%) cases were acquired in South America (Brazil, Guyana, and Peru) and three (6.1%) cases in Mexico. Information regarding region of acquisition was missing for 504 (38.8%) imported cases.
In the United States, seven jurisdictions accounted for 52.4% of the reported cases: New York City (n = 182), California (n = 128), Texas (n = 91), Maryland (n = 78), Illinois (n = 77), New Jersey (n = 63), and Georgia (n = 61) (Figure 1). Despite the decrease in the number of cases from 2007 to 2008, certain states reported an increase. The states with the most notable change in reported malaria cases in 2008 were Iowa, Georgia, and Maryland. The number of cases reported in Iowa increased from three cases in 2007 to 12 cases in 2008 (p = 0.009). Cases in Georgia increased by almost 33%, from 46 cases reported in 2007 to 61 cases in 2008 (p = 0.02). Maryland reported an increase in cases from 67 in 2007 to 78 in 2008 (p = 0.04).
Imported Malaria by Resident Status
Among 703 imported malaria cases in patients with known resident status, 527 (75.0%) occurred among U.S. residents and 176 (25.0%) among residents of other countries. Among the 527 imported malaria cases in U.S. residents, 386 (73.2%) were acquired in Africa, 92 (17.4%) were acquired in Asia, and 35 (6.6%) were acquired in the Americas (Table 4). Among the 176 imported cases in foreign residents, 108 (61.4%) were acquired in Africa. Among the 157 foreign cases in patients for whom purpose of travel was known, 77 (49.0%) occurred in recent immigrants or refugees, and 52 (33.1%) occurred in patients who were visiting friends and relatives in the United States.
Estimated Relative Case Rates for U.S. Residents
In 2008, the countries with the lowest estimated malaria case rates for U.S. travelers were Mexico and South Korea (Figure 2). Other countries with low estimated relative case rates included the Philippines, Guatemala, and Iraq. In many of these countries, malaria risk areas are localized in small parts of the country. Countries with estimated relative case rates in the middle range included India, Honduras, Haiti, and Pakistan, which have more homogenous malaria transmission throughout the country. Estimated relative case rates were highest in West African countries, including Guinea, Togo, Côte d'Ivoire, and Nigeria. These high estimated case rates reflect not only widespread transmission areas but likely also higher transmission intensity.
Interval Between Arrival in the United States and Illness Onset
The interval between the date of arrival in the United States and onset of illness and the infecting Plasmodium species were known for 527 (41.0%) imported malaria cases (Table 5). Symptoms began before arrival in the United States in 73 (12.8%) patients and after arrival in 498 (87.2%) patients. Onset of malaria symptoms occurred <1 month after arrival in 316 (96.3%) of the 328 patients with P. falciparum malaria and in 56 (54.4%) of the 103 patients with P. vivax cases (Table 5).
Imported Malaria Among U.S. Military Personnel
In 2008, a total of 19 cases of imported malaria were reported among U.S. military personnel. Seventeen patients reported travel to Afghanistan, and two reported travel to Iraq and South Korea. Information on infecting species was known for 18 cases; all but two cases were P. vivax. Among the 19 total cases, 13 occurred in person who reported having taken an appropriate drug for primary chemoprophylaxis; three patients reported adherence to the prescribed drug regimen. Among the 16 patients infected with P. vivax, two reported taking primaquine, which is recommended for presumptive antirelapse therapy. These cases were reported by state health departments and do not include all cases that might have occurred among military personnel.
Chemoprophylaxis Use Among U.S. Civilians
Information concerning chemoprophylaxis use and travel area was known for 480 (94.5%) of the 508 U.S. civilians who had imported malaria. Among the 480, a total of 136 (28.3%) had taken chemoprophylaxis. Among these, 97 (71.3%) had taken a CDC-recommended medication for the area visited, 19 (14.0%) had not taken a CDC-recommended medication, and data for the specific drug taken were missing for 20 (14.7%) travelers. A total of 39 (40.2%) patients who received CDC-recommended chemoprophylaxis had taken mefloquine; 29 (29.9%) had taken doxycycline; 23 (23.7%) had taken atovaquone-proguanil; and two (2.1%) who had traveled only in areas where chloroquine-resistant malaria has not been documented had taken chloroquine. An additional four patients had taken a combination of two malaria drugs recommended by CDC for the specific travel region.
Malaria Infection After Recommended Prophylaxis Use
A total of 97 U.S. civilians contracted malaria after taking a recommended antimalarial drug for chemoprophylaxis. Of these, 20 (20.6%) reported complete adherence with the drug regimen, and 68 (70.1%) reported nonadherence; adherence was unknown for nine (9.2%) patients. Information regarding infecting species was available for 90 (92.8%) patients who had taken a recommended antimalarial drug and was undetermined for seven patients.
P. vivax or P. ovale Cases
Among the 97 patients who had malaria diagnosed after recommended chemoprophylaxis use, 28 (28.9%) were infected with P. vivax, and four (4.1%) were infected with P. ovale. Among the 32 total cases of P. vivax or P. ovale, eight (25%) occurred >45 days after arrival in the United States. These cases were consistent with relapsing infections and do not indicate primary prophylaxis failures. Information on 12 cases was insufficient (i.e., missing data regarding symptom onset or return date) to assess whether they were relapsing infections. A total of 11 cases occurred ≤45 days after the patient returned to the United States. Eight of the 11 patients did not adhere to their malaria chemoprophylaxis regimen. The three patients who reported adherence to the chemoprophylaxis regimen had traveled to Africa and had all taken atovaquone-proguanil recommended for malaria chemoprophylaxis. Possible explanations for these cases include inappropriate dosing, unreported nonadherence, drug malabsorption, an early relapse from hypnozoites established at the start of the trip, or emerging parasite resistance.
P. falciparum and P. malariae Cases
The 58 malaria cases among patients who had taken a recommended antimalarial drug for chemoprophylaxis included 56 P. falciparum cases and two P. malariae cases. All of the 56 P. falciparum cases were acquired in Africa. Forty-three (76.8%) of these patients reported nonadherence to the antimalarial drug regimen, and six patients had no adherence information available. In seven (12.5%) cases, patients reported adherence with antimalarial chemoprophylaxis, all of whom had traveled to Africa. Two patients took doxycycline, and seven patients took atovaquone-proguanil. Both P. malariae cases were acquired in Africa and only one reported adherence to the antimalarial drug regimen (atovaquone-proguanil).
Purpose of Travel
Purpose of travel to areas in which malaria is endemic was reported for 459 (90.4%) of the 508 U.S. civilians with imported malaria. Although travelers could report multiple reasons for travel, the largest proportion (65.3%) was VFR travelers; the second and third highest proportions (7.9% and 7.1%) were persons who had traveled as missionaries or on business, respectively (Table 6). The proportions of reason for travel in all categories in 2008 were similar to 2007, except for tourism; the proportion of patients with malaria who traveled as tourists decreased by 38% from 2007 to 2008 (p<0.001). A significant association was found between purpose for travel and prophylaxis use among VFR and business/tourist travelers. Among VFR travelers, 21.7% (72 of 332) used prophylaxis, compared with 42.6% (29 of 68) of business/tourist travelers (p<0.001). A significant association also was found between VFR travelers and geographic region of infection acquisition, specifically in Africa, where 79.5% (264 of 332) of VFR travelers acquired malaria, compared with 62.6% (122 of 195) of persons traveling for other reasons (p<0.001). No significant association was noted for VFR travelers (or other non-VFR travelers) who acquired infections in other geographic regions. In addition, 72.9% (242 of 332) VFR travelers acquired a P. falciparum infection, compared with 57.4% (112 of 195) of non-VFR travelers (p<0.001). No significant association was found between non-VFR travelers and species type.
Malaria by Age
Among the 1,274 cases among patients for whom age was known, 241 (18.9%) cases occurred in patients aged <18 years, 995 (78.1%) in patients aged 18--64 years, and 38 (3.0%) in patients aged ≥65 years. Although the majority of cases occur in persons aged 18--64 years, cases in children are of particular interest because preventive health care for most children is determined by parents or guardians. Among the 241 cases in patients aged <18 years, 86 (35.7%) cases occurred among U.S. civilian children (including one congenital case), 68 (28.2%) among children of foreign citizenship, and 87 (36.1%) among children of unknown resident status.
Sixty-two (72.1%) of the cases among U.S. civilian children for whom country of exposure was known were attributed to travel to Africa. Among the 86 cases in U.S. civilian children, four (4.6%) children were aged <24 months, 12 (14.0%) were aged 24--59 months, 33 (38.4.0%) were aged 5--12 years, and 37 (43.0%) were aged 13--17 years. Among the 76 U.S. civilian children for whom reason for travel was known, 65 (85.5%) were reported as visiting friends and relatives; the remaining 11 cases (14.5%) were attributed to missionary or student travel. Twenty-four (29.3%) of the 82 children for whom chemoprophylaxis information was known were reported as having taken chemoprophylaxis, of whom 14 (58.3.3%) had taken an appropriate regimen; however, only three (21.4%) reported complete adherence
Severe Malaria
CDC categorizes severe malaria cases according to WHO criteria for diagnosing severe P. falciparum infection, as well as on the basis of receipt of certain treatments (i.e., artesunate, quinidine, or an exchange blood transfusion). WHO criteria include reports of any of the following: neurological symptoms, renal failure, severe anemia (hemoglobin [Hb] level <7 g/dL), ARDS, jaundice, or parasitemia (12). In 2008, to facilitate uniform reporting of certain variables, CDC revised the malaria case surveillance report form (i.e., by including a section to quantify parasitemia and adding a check box for artesunate).
Among the 1,298 reported cases, 117 (9%) were classified as severe malaria, of which two were fatal cases. Although approximately 70% of all cases were identified in U.S. civilians, no association was found between risk for developing severe malaria and sex (p = 1.0), age (p = 0.54), resident status (p = 0.72), or prophylaxis use (p = 0.15). The predominant species among the severe cases was P. falciparum (82%). A significant association between severe malaria and species type was found. In patients for whom reason for travel was known, 48% of the cases were in VFR travelers, of whom 86% reported travel in countries in West Africa, and 93% were identified as P. falciparum infections. A significant association was found between VFR travel and severe malaria. Among VFR travelers, 13.8% (56 of 405) developed severe disease, compared with 6.8% (61 of 893) of persons traveling for other reasons (p<0.001). The majority of severe cases (73%) occurred in patients aged 18--64 years, and 24% occurred in children aged <18 years, three of whom were aged <3 years. Twenty-one (7%) patients reported having taken a recommended chemoprophylaxis; however, only five reported complete adherence to the drug regimen, all of whom were using doxycycline for chemoprophylaxis. Although patients could have had multiple clinical complications associated with an infection, the largest proportion (33.3%) of patients experienced severe anemia followed by renal failure (28.3%). Twenty-four cases were treated with artesunate administered intravenously (IV) provided by CDC through an investigational new drug protocol.
Malaria During Pregnancy
A total of 14 cases of malaria were reported among pregnant women in 2008, representing 3.2% of cases among all women (N = 433). No significant difference in cases was noted between pregnant women and women overall in terms of species type, reason for travel, or region of infection acquisition. Nine (64.3%) of the 14 cases occurred among U.S. civilians; seven patients reported travel to Africa, one to India, and one to Nicaragua. Among the six women for whom reason for travel was known, five reported visiting friends and relatives, and one reported travel because of business. Among the nine cases of malaria reported among U.S. civilian pregnant women, none of the women reported taking malaria chemoprophylaxis. Although approximately 70% of the pregnant women were diagnosed with a P. falciparum infection, none had severe malaria. No information was available on the birth outcomes.
Selected Malaria Case Reports
Congenital Malaria
One congenital case of malaria, caused by transmission of parasites from mother to child during pregnancy or perinatally during labor, was reported in 2008. On January 10, 2008, an infant aged 1 month was admitted to the hospital for fever and suspected sepsis. After 72 hours, the infant was released but was then readmitted after 5 days of persistent fever. After investigation, the health-care provider reported that the mother had moved to the United States from Cambodia in September 2007 and had experienced at least three episodes of malaria; the most recent infection had occurred in 2007. The infant had been born via vaginal delivery without complications. At the time of readmission, the infant was anemic but hemodynamically stable, and P. vivax parasites were observed on a peripheral blood film. The infant promptly received quinine and clindamycin followed by a transfusion of packed red blood cells. Quinine and clindamycin were discontinued the next day, and treatment was completed with chloroquine. The infant was observed for 24 hours after the last dose of chloroquine, continued to improve, and was discharged from the hospital.
Cryptic Malaria
One case reported in 2008 was categorized as cryptic malaria because epidemiologic investigations failed to identify a plausible mode of acquisition. On July 25, 2008, a man aged 64 years sought treatment at a Maryland hospital for recurrent falls and lightheadedness. Soon after admission, he was transferred to the intensive care unit (ICU) for acute renal failure, severe thrombocytopenia, and fever. Thin and thick blood films revealed P. falciparum parasites, with a parasitemia of >80% (confirmed). Treatment included transfusion of 2 units of packed red blood cells, IV quinidine, and oral doxycycline.
Probable routes of transmission were investigated. The patient stated that he had not traveled outside of the United States during the preceding 6 months. In November 2007, he and his family took a Caribbean vacation cruise, which stopped in the islands of St. Maarten, Dominica, Barbados, St. Kitts, St. Thomas, Antigua, and Puerto Rico; malaria is not endemic in any of these areas. The man did not recall getting any mosquito bites while on vacation. Testing at CDC revealed that the infecting parasites had a chloroquine-resistant phenotype most commonly found in those from Africa or Asia. The man reported no history of blood transfusion or IV drug use. Mosquito trappings conducted around his home and workplace revealed no Plasmodium-infected mosquitoes. No additional malaria cases were reported near his home, and he did not work in a profession that placed him in contact with blood or blood products. The origin of the infection remains undetermined.
Simian Malaria (P. knowlesi)
On October 17, 2008, a woman aged 50 years with no history of malaria who was born in the Philippines but had lived in the United States for 25 years returned to the Philippines to visit friends and relatives. While there, she stayed on the island of Palawan in a cabin at the edge of a forested area known to be a habitat for long-tailed macaques. She had not taken malaria chemoprophylaxis and had not used any mosquito-avoidance measures, both of which are recommended for travelers to this area.
The woman returned to the United States on October 30 and noted the onset of a headache. Fever and chills followed, and symptoms persisted for several days, after which she sought medical attention. Thick and thin blood films were ordered, and a laboratory technician made the initial (incorrect) diagnosis of babesiosis. After a review by the laboratory supervisor the next morning, the diagnosis was changed to malaria with 2.9% parasitemia. However, the atypical appearance of the Plasmodium species prevented a species-specific diagnosis. The woman was treated successfully with atovaquone-proguanil and primaquine for undetermined Plasmodium species.
Blood and two stained films were sent to the New York Wadsworth Center Parasitology Reference Laboratory for confirmation of malaria and molecular determination of species by PCR. The laboratory identified the Plasmodium species; however, PCR testing did not yield results consistent with any of the four species of Plasmodium known to infect humans. Additional Plasmodium genus matching detected infection with P. knowlesi (13).
Deaths Attributed to Malaria
Two deaths attributable to malaria were reported in 2008. Clinical and laboratory data were limited.
Case 1. A woman aged 32 years traveled to Ethiopia for 4 months and returned to the United States on September 9, 2008. She had not taken any recommended chemoprophylaxis. She reported experiencing fever, chills, and nausea on September 23, and she sought medical attention the next day. No conclusive diagnosis was made, and she was treated with ciprofloxacin and doxycycline. On September 29, she was admitted to the emergency department (ED) with a P. vivax malaria infection (0.75%--1.5% parasitemia), which was later confirmed by PCR at CDC. Although the woman appeared to have uncomplicated malaria, she was admitted to the ICU for monitoring of thrombocytopenia (platelet count = 20,000/mm3). In the ICU, she was treated with IV quinidine and clindamycin. On September 30, IV doxycycline, a higher dose of clindamycin, and 2 units of platelets were administered. The same day, she developed ARDS and was treated with IV methylprednisolone and an exchange transfusion of 7 units. Quinidine treatment was stopped and replaced with oral chloroquine. She began receiving mechanical ventilation on October 1.
The same day, the hospital contacted CDC to request IV artesunate, which was provided. On October 2, the parasitemia level was 0.5%; the woman received her second exchange transfusion of 10 units. Although the various medical interventions reduced the parasite level, her lung function continued to deteriorate. (Although rapid decline in lung function is not unexpected in severe P. vivax cases, it rarely occurs.) She died on October 3.
Case 2. In April 2008, a Nigerian man aged 47 years returned to the United States after a 3-week trip to Nigeria to visit friends and relatives. On April 28, the man was found unresponsive in his car and was taken to the ED by emergency medical services, where he was pronounced dead 10 minutes later. The city medical examiner performed an autopsy and reported that the death was likely caused by malaria. Toxicologic serum assays were consistent with pyrimethamine and chloroquine ingestion, and a postmortem blood examination revealed P. falciparum parasites.
Discussion
A total of 1,298 cases of malaria were reported to CDC with onset in 2008, a 13.8% decrease from the 1,505 cases reported in 2007. Several possible explanations might account for the decrease in reported malaria cases from 2007 to 2008, including changes in worldwide travel patterns to countries in which malaria is endemic, decreased transmission, changes in case monitoring, or improved prevention interventions. The decrease is unlikely a result of a decrease in travel between the United States and malarious countries because from 2007 to 2008, cumulative travel to and from the United States increased by an estimated 1% (6).
An additional possible explanation for the decrease in U.S. cases includes worldwide efforts to decrease the transmission of malaria in many countries where the disease is endemic. These malaria prevention and treatment programs have resulted in increased coverage with effective intervention in some countries and a decrease in associated malaria morbidity (14). Such efforts are supported by the Global Fund for AIDS, Tuberculosis, and Malaria; the U.S. President's Malaria Initiative; and the World Bank Booster program and led by National Malaria Control Programs working in conjunction with WHO, other United Nations agencies, nongovernmental agencies, community organizations, academia, and the private sector. With continued prevention and treatment efforts and reduced transmission in countries with endemic malaria, the number of cases in the United States might continue to decrease; however, whether the decrease will continue is uncertain.
CDC relies on reporting from each state and territory to compile national data on malaria cases; therefore, clinicians and health-care facilities should report all malaria cases promptly so that annual trends in cases can be assessed and monitored. The United States remains at risk for reintroduction of malaria because the mosquito vector, conducive environmental conditions, or both are present in all U.S. states and territories. Therefore, clinicians and health-care facilities must continue to report all malaria cases to their respective state public health authorities.
NMSS is the primary source for malaria-specific epidemiologic and clinical information (e.g., travel history, chemoprophylaxis use, and treatment regimens). However, since 2001, an annual average of 7% more cases have been reported to NNDSS than to NMSS (17); in 2008, 445 (34%) more cases were reported to NNDSS than NMSS, a significant increase in cases compared with 2007. Because of incomplete reporting, important variables are missing for many cases, which compromises efforts to follow trends in malaria cases and prevent infections. CDC is integrating NMSS and NNDSS into a single electronic system for reporting malaria in the United States. Once in place, the level of incomplete reporting should decrease.
The proportion of epidemiologic and clinical details provided for cases reported through NMSS was similar in 2008 and 2007, although 5% fewer cases included information on species type. Case details such as species type and geographic region of infection acquisition can be used to identify possible changes in the location of species transmission or to decrease misidentification of species. For example, the first simian case of malaria identified in the United States was initially identified as a Babesia species infection in Malaysia; in addition, some P. knowlesi cases are misdiagnosed as P. malariae (18). Microscopy is still considered the best method for diagnosing malaria; however, PCR testing can be used for species confirmation. CDC can provide assistance to reference laboratories or health departments that do not perform PCR testing. Increasing the proportion of cases with complete epidemiologic and clinical information will improve identification of geographic trends in risk for infection.
Improved implementation of malaria prevention measures among travelers (especially among VFR travelers) might decrease the number of total malaria cases in the United States. For example, health-care professionals might provide information regarding malaria prevention more consistently, and more travelers might adhere to recommended prevention measures. In 2008, VFR travelers accounted for the majority of reported cases among patients for whom reason for travel was known. Among these, approximately 20% were children aged <18 years. Foreign-born U.S. civilians must be informed that acquired immunity wanes quickly when exposure to malaria is not continuous; therefore, prophylaxis is needed when returning to malarious areas. In addition, children of foreign-born U.S. civilians who were born in the United States do not have immunity to malaria and are at risk for infection (16). A total of 70% of infected VFR travelers had not been taking any chemoprophylaxis or had not been taking a recommended medication for chemoprophylaxis. Therefore, better health communication efforts are needed to inform the traveling population, particularly VFR travelers, about malaria prevention.
The number of severe malaria cases increased from 57 in 2007 to 117 cases in 2008; however, no conclusions can be drawn about this increase because the case report form had been modified to facilitate uniform reporting. As in previous years, cases in VFR travelers represented approximately half of severe cases. Among these VFR travelers with severe malaria, 79% had traveled to countries in West Africa. Furthermore, VFR travelers were almost two times more likely to develop a severe case of malaria than persons traveling for other reasons. This difference might be because many of these cases were P. falciparum infections, which are often more severe. Severe malaria in VFR travelers also might have resulted from a delay in seeking treatment after illness onset; however, the surveillance data in this report cannot be used to quantify the time delayed in seeking care. Some travelers might assume they might have partial immunity, and the consequences of a delay in seeking medical care after illness onset are substantial. Clinicians should emphasize malaria prevention for VFRs travelers to countries in West Africa.
Malaria surveillance data can be used to detect prophylaxis failures that might indicate emergence of drug resistance. However, approximately 71.7% of imported malaria cases among U.S. residents for whom prophylaxis use was known occurred among patients who did not take prophylaxis, took prophylaxis but not the recommended prophylaxis, or took a recommended prophylactic regimen incorrectly. Information on prophylactic drug levels among patients who reported adherence to a recommended regimen is not generally available; therefore, differentiating inaccurate reporting of adherence from malabsorption of the antimalarial drug or emerging drug resistance is difficult. There is no conclusive evidence to indicate failure of a particular chemoprophylactic regimen. Health-care providers are encouraged to contact CDC promptly when they suspect a chemoprophylaxis failure, which enables CDC, if possible, to measure blood drug levels and in vitro parasite response to antimalarial drugs.
Cases among patients who were traveling on military duty to an area where malaria is endemic are reported by local and state health departments and private health clinicians. In 2008, a total of 19 cases of imported malaria were reported among U.S. military personnel to CDC. However, in a report published by the Armed Forces Health Surveillance Center, 83 U.S. military members were diagnosed or reported with a malaria infection (20). This number includes active and reserve members of the Army, Navy, Air Force, and Marine Corps. Among the 83 cases, 33 cases were diagnosed or reported in U.S. military facilities in the United States (Fort Bragg, North Carolina; Fort Lewis, Washington; Bethesda, Maryland; Philadelphia, Pennsylvania; Agana, Guam; Camp Lejeune, North Carolina; and South Carolina). These 33 cases would have been counted as U.S. cases in NMSS. Assuming that 19 of the 33 cases are included in this report, 14 cases were not accounted for in this analysis. The difference in case numbers might be a result of differences in the diagnostic and reporting practices of military and civilian health systems and lack of attribution of military duty as the reason for travel by some military personnel. By partnering with the Department of Defense, CDC will attempt to include all malaria cases among military members who receive their diagnosis and treatment in the United States in future surveillance reports.
Fourteen cases were reported in pregnant women, which is a 41% decrease from 2007. Of the 14 cases in pregnant women, nine cases were identified in U.S. civilians; none reported taking any chemoprophylaxis before travel. Malaria during pregnancy poses a substantial risk for both maternal and perinatal morbidity and mortality (21), as demonstrated by the congenital malaria case reported in 2008. Pregnant travelers should be counseled to avoid travel to malarious areas. If deferral of travel is not feasible, pregnant women should be informed that the risks for malaria greatly outweigh those associated with prophylaxis and that safe chemoprophylaxis regimens are available. Specific guidance for pregnant travelers is available at http://www.cdc.gov/malaria/travel/drugs_pregnant_public.htm.
Two fatal cases were reported in 2008, one P. falciparum case and one P. vivax case. Clinicians should be aware that nonfalciparum species can cause severe illness and should therefore emphasize prevention of all types of malaria when counseling patients before travel. The differential diagnosis of fever in a person who has returned from travel should always include malaria as one of the primary possibilities. Signs and symptoms of malaria often are nonspecific but typically include fever. Other symptoms include headache, chills, increased sweating, back pain, myalgia, diarrhea, nausea, vomiting, and cough. Prompt diagnosis requires that malaria be included in the differential diagnosis of illness in a febrile person with a history of travel to a malarious area. Clinicians should ask all febrile patients for a travel history, including information regarding international visitors, immigrants, refugees, migrant laborers, and other international travelers. Any delay in the diagnosis and treatment of malaria can result in complications, regardless of the effectiveness of the treatment regimen. IV artesunate is highly effective in severe cases if treatment is started promptly. This medication is stocked at eight quarantine stations around the United States and can be rapidly shipped when needed. In addition, precise guidelines and eligibility requirements must be met to enroll a patient in the treatment protocol. Artesunate is provided free to hospitals, on request and on an emergency basis, by the CDC drug service or by one of the CDC quarantine stations. Physicians who administer the drug to patients must notify CDC of any adverse event that occurs after administration and comply with the investigational new drug protocol (19). To enroll a patient with severe malaria in this treatment protocol, during regular business hours, health-care providers should telephone the CDC Malaria Hotline (Table 7). During evenings, weekends, and holidays, callers should telephone the CDC Emergency Operations Center (Table 7) and ask to speak to a CDC Malaria Branch clinician.
Prompt treatment of suspected malaria is essential because patients with P. falciparum infection are at risk for developing life-threatening complications soon after the onset of illness. Ideally, therapy for malaria should be initiated immediately after the diagnosis has been made. Treatment should be determined on the basis of the infecting Plasmodium species, the probable geographic origin of the parasite, level of parasitemia, and the clinical status of the patient (21). If a diagnosis of malaria is suspected but cannot be confirmed or if a diagnosis of malaria is confirmed but the species cannot be determined, antimalarial treatment that is effective against P. falciparum should be initiated. Resistance of P. falciparum to chloroquine generally occurs worldwide, with the exception of a few geographic regions (e.g., Mexico and Central America). Therefore, therapy for presumed P. falciparum malaria should entail the use of a drug effective against such resistant strains (22).
Health-care providers should be familiar with prevention, recognition, and treatment of malaria and are encouraged to consult appropriate sources for malaria prevention and treatment recommendations (Table 7). Physicians seeking assistance with the diagnosis (including telediagnosis) or treatment of patients with suspected or confirmed malaria should call the CDC Malaria Hotline during regular business hours or the CDC Emergency Operations Center during evenings, weekends, and holidays (Table 7). Information also is available from CDC online (http://www.cdc.gov/malaria/diagnosis_treatment/treatment.htm). These resources are intended for use by health-care providers only.
Detailed recommendations for preventing malaria are available to the general public online (http://www.cdc.gov/travel/diseases.htm/malaria). Additional information on malaria prevention recommendations is also available through the online CDC malaria map application (http://www.cdc.gov/malaria/map). This interactive map allows users to search or browse countries, cities, and place names and get information about malaria in a particular location and to see recommended malaria prevention medicines for a certain area. In addition, CDC biannually publishes recommendations in Health Information for International Travel (i.e., The Yellow Book) (2), which is available and updated on the CDC Travelers' Health website and is also available for purchase (Table 7).
Acknowledgments
The authors acknowledge the state, territorial, and local health departments; health-care providers; and laboratories that reported this information to CDC.
References
- World Health Organization. World malaria report 2009. WHO Press. 2009.
- CDC. Health information for international travel 2008. Atlanta, GA: CDC; 2008.
- Pan American Health Organization. Report for registration of malaria eradication from United States of America. Washington, DC: Pan American Health Organization; 1969.
- CDC. Multifocal autochthonous transmission of malaria---Florida, 2003. MMWR 2004;53:412--3.
- CDC. National notifiable diseases surveillance system. November 10, 2009. Available at http://www.cdc.gov/ncphi/disss/nndss/nndsshis.htm. Accessed April 19, 2010.
- World Tourism Organization. Estimates of travel [CD-ROM]. Madrid, Spain: World Tourism Organization; 2009.
- Council of State and Territorial Epidemiologists position statement no. 09-ID-47. Available at http://www.cdc.gov/ncphi/disss/nndss/casedef/malaria_current.htm. Accessed April 19, 2010.
- World Health Organization. Terminology of malaria and of malaria eradication: report of a drafting committee. Geneva, Switzerland: World Health Organization; 1963:32.
- CDC. Malaria surveillance---United States, 2007. MMWR 2009;58(No. SS-2).
- CDC. Malaria rapid diagnostic test. MMWR 2007;56:686.
- BinaxNOW Malaria [Package insert]. Scarborough, ME: Inverness Medical Professional Diagnostics; 2007.
- World Health Organization. Diagnosis and management of severe falciparum malaria. Geneva, Switzerland: World Health Organization; 2000. Available at http://whqlibdoc.who.int/hq/2000/who_cds_cpe_smt_2000.4_part1.pdf. Accessed April 19, 2010.
- CDC. Simian malaria in a U.S. traveler---New York, 2008. MMWR 2009;58;229--32
- Campbell CC. Malaria control---addressing challenges to ambitious goals. N Engl J Med 2009;361:522--3.
- World Health Organization, UNICEF. World malaria report 2005. Geneva, Switzerland: WHO Press; 2005. Available at http://www.rollbackmalaria.org/wmr2005. Accessed April 19, 2010.
- Fulford M, Keystone JS. Health risks associated with visiting friends and relatives in developing countries. Curr Infect Dis Rep 2005;7:48--53
- Hwang J, McClintock S, Kachur SP, Slutsker L, Arguin P. Comparison of national malaria surveillance system with the national notifiable disease surveillance system in the United States. J Public Health Manag Pract 2009;15:345--51
- Cox-Singh J, Davis TM, Lee KS, et al. Plasmodium knowlesi malaria in humans is widely distributed and potentially life threatening. Clin Infect Dis 2008;46:165--71.
- CDC. New medication for severe malaria available under an investigational new drug protocol. MMWR 2007;56:769--70.
- Armed Forces Health Surveillance Center. Update: malaria, U.S. armed forces, 2008. Medical Surveillance Monthly Report (MSMR) 2009;16(1):8-11.
- Griffith KS, Lewis LS, Mali S, Parise ME. Treatment of malaria in the United States: A systematic review. JAMA 2007;297:2264--76.
- Baird JK. Effectiveness of antimalarial drugs. N Engl J Med 2005;352:1565--77.
* For confirmation of malaria species, refer to the CDC Division of Parasitic Diseases and Malaria diagnostic website (DPDx) at http://www.dpd.cdc.gov/dpdx, or e-mail the diagnostic request to dpdx@cdc.gov.
FIGURE 1. Number of malaria cases (N = 1,298), by state or territory in which case was diagnosed --- United States, 2008
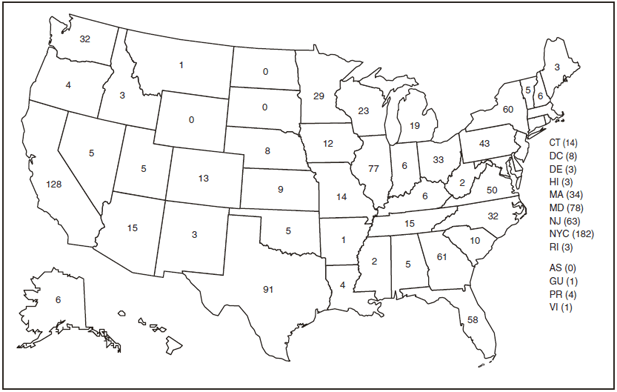
Abbreviations: AS = American Samoa; GU = Guam; PR = Puerto Rico; VI = U.S. Virgin Islands.
Alternate Text: Figure 1 is a U.S. map showing the number of malaria cases (N = 1,298) in 2008, by state or territory in which the case was diagnosed: Alabama, 5 cases; Alaska, 6 cases; Arizona, 15 cases; Arkansas, 1 case; California, 128 cases; Colorado, 13 cases; Connecticut, 14 cases; Delaware, 3 cases; District of Columbia, 8 cases; Florida, 58 cases; Georgia, 61 case; Hawaii, 3 cases; Idaho, 3 cases; Illinois, 77 cases; Indiana, 6 cases; Iowa, 12 cases; Kansas, 9 cases; Kentucky, 6 cases; Louisiana, 4 cases; Maine, 3 cases; Maryland, 78 cases; Massachusetts, 34 cases; Michigan, 19 cases; Minnesota, 29 cases; Mississippi, 2 cases; Missouri, 14 cases; Montana, 1 case; Nebraska, 8 cases; Nevada, 5 cases; New Hampshire, 6 cases; New Jersey, 63 cases; New Mexico cases; New York, 60 cases; New York City, 182 cases; North Carolina, 32 cases; North Dakota, 0 cases; Ohio, 33 cases; Oklahoma, 5 cases; Oregon, 32 cases; Pennsylvania, 43 cases; Rhode Island, 3 cases; South Carolina, 10 cases; South Dakota, 0 cases; Tennessee, 15 cases; Texas, 91 case; Utah, 3 cases; Vermont, 5 cases; Virginia, 50 cases; Washington, 4 cases; West Virginia, 2 cases; Wisconsin, 23 cases; Wyoming, 0 cases; American Samoa, 0 cases; Guam, 1 case; Puerto Rico, 4 cases; U.S. Virgin Islands, 1 case.
FIGURE 2. Imported malaria cases and estimated relative case rates among U.S. residents, by country of acquisition --- 2008
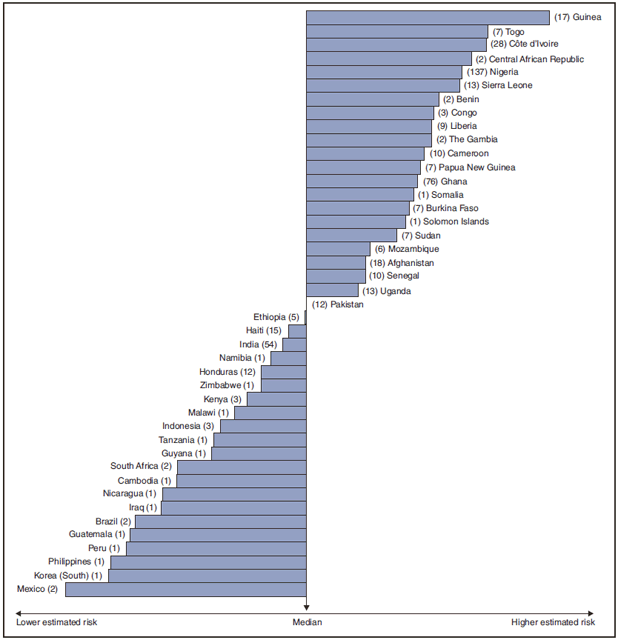
* Using estimates of travel volume for U.S. travelers to each country from which cases of malaria were acquired and the number of cases among U.S. travelers attributable to each country, a case rate was estimated for each country. Data used to estimate country-specific relative case rates were extrapolated from World Tourism Organization estimates of annual numbers of U.S. travelers to specified countries (6). Relative case rates were determined by dividing the individual country-specific case rates by the median individual country-specific case rate. The number of cases of malaria among U.S. civilian travelers attributable to each country is displayed next to the country name in parentheses.
Alternate Text: Figure 2 is a horizontal bar chart showing the imported malaria cases and estimated relative case rates among U.S. residents in 2008, by country of acquisition. The estimated case rates from lowest to highest estimated risk are as follows, as well as the number of cases of malaria among U.S. civilian travelers attributable to each country: Mexico, lowest estimated risk (2 cases), Korea (South) (1 case), Philippines (1 case), Peru (1 case), Guatemala (1 case), Brazil (2 cases), Iraq (1 case), Nicaragua (1 case), Cambodia (1 case), South Africa (2 cases), Guyana (1 case), Tanzania (1 case), Indonesia (3 cases), Malawi (1 case), Kenya (3 cases), Zimbabwe (1 case), Honduras (12 cases), Namibia (1 case), India (54 cases), Haiti (15 cases), Ethiopia (5 cases), Pakistan (12 cases), Uganda (13 cases) Senegal (10 cases), Afghanistan (18 cases), Mozambique (6 cases), Sudan (7 cases), Solomon Islands (1 case), Burkina Faso (7 cases), Somalia (1 case), Ghana (76 cases), Papua New Guinea (7 cases), Cameroon (10 cases), The Gambia (2 cases), Liberia (9 cases), Congo (3 cases), Benin (2 cases), Sierra Leone (13 cases), Nigeria (137 cases), Central African Republic (2 cases), Côte d’Ivoire (28 cases), Togo (7 cases), Guinea, highest estimated risk (17 cases).
|
TABLE 7. Sources for malaria prophylaxis, diagnosis, and treatment recommendations |
|||
|---|---|---|---|
|
Type of information |
Source |
Availability |
Contact information |
|
Prophylaxis |
CDC Traveler's Health website (includes online access to Health Information for International Travel [The Yellow Book]) |
24 hrs/day |
|
|
Prophylaxis |
Health Information for International Travel (The Yellow Book) |
Order from: Elsevier, Health Sciences Division Order Fulfillment11830 Westline Industrial DriveSt. Louis, MO 63146 |
800-545-2522 or http://www.elsevier.com |
|
Prophylaxis |
CDC Malaria Map Application |
24 hrs/day |
|
|
Diagnosis |
CDC Division of Parasitic Diseases and Malaria diagnostic internet site (DPDx) |
24 hrs/day |
|
|
Diagnosis |
CDC Division of Parasitic Diseases and Malaria diagnostic CD-ROM (DPDx) |
Order by e-mail from CDC Division of Parasitic Diseases |
dpdx@cdc.gov |
|
Treatment* |
CDC Malaria Branch |
9:00 a.m.-- 5:00 p.m. (EST), Monday-- Friday |
770-488-7788* |
|
5:00 p.m.--9:00 a.m. (EST), Monday--Friday; all day weekends and holidays |
770-488-7100* This is the number for the CDC Emergency Operations Center. Ask staff member to page person on call for Malaria Branch. http://www.cdc.gov/malaria/diagnosis_treatment/treatment.htm |
||
|
* Telephone number is intended for use by health-care professionals only. |
|||
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All MMWR HTML versions of articles are electronic conversions from typeset documents.
This conversion might result in character translation or format errors in the HTML version.
Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr)
and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S.
Government Printing Office (GPO), Washington, DC 20402-9371;
telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to
mmwrq@cdc.gov.


