Prevention and Control of Haemophilus influenzae Type b Disease: Recommendations of the Advisory Committee on Immunization Practices (ACIP)
Corresponding preparer: Elizabeth C. Briere, MD, National Center for Immunization and Respiratory Diseases, CDC, 1600 Clifton Road NE, MS C-09, Atlanta, GA 30333. E-mail: ebriere@cdc.gov.
Summary
This report compiles and summarizes all recommendations from CDC's Advisory Committee on Immunization Practices (ACIP) regarding prevention and control of Haemophilus influenzae type b (Hib) disease in the United States. As a comprehensive summary of previously published recommendations, this report does not contain any new recommendations; it is intended for use by clinicians, public health officials, vaccination providers, and immunization program personnel as a resource. ACIP recommends routine vaccination with a licensed conjugate Hib vaccine for infants aged 2 through 6 months (2 or 3 doses, depending on vaccine product) with a booster dose at age 12 through 15 months. ACIP also recommends vaccination for certain persons at increased risk for Hib disease (i.e., persons who have early component complement deficiencies, immunoglobulin deficiency, anatomic or functional asplenia, or HIV infection; recipients of hematopoietic stem cell transplant; and recipients of chemotherapy or radiation therapy for malignant neoplasms). This report summarizes current information on Hib epidemiology in the United States and describes Hib vaccines licensed for use in the United States. Guidelines for antimicrobial chemoprophylaxis of contacts of persons with Hib disease also are provided.
Introduction
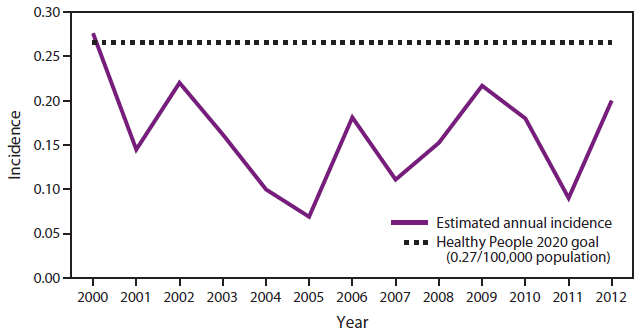
Before 1985, Haemophilus influenzae type b (Hib) was the leading cause of bacterial meningitis and a common cause of other invasive diseases (e.g., epiglottitis, pneumonia, septic arthritis, cellulitis, purulent pericarditis, and bacteremia) among U.S. children aged <5 years (1). Meningitis occurred in approximately two thirds of children with invasive Hib disease; 15%–30% of survivors had hearing impairment or severe permanent neurologic sequelae. Approximately 4% of all cases were fatal (2). The first polysaccharide Hib vaccine was introduced in the United States in 1985, followed by conjugate Hib vaccines in 1987 and 1989. During 1989–2000, the annual incidence of invasive Hib disease in children aged <5 years decreased by 99%, to less than one case per 100,000 children (3–7). During 2000–2012, the average annual incidence rate of invasive Hib disease in children aged <5 years in the United States remained below the Healthy People 2020 goal of 0.27/100,000 (8) (data available at http://www.cdc.gov/abcs/reports-findings/surv-reports.html) (Figures 1 and 2). Studies have demonstrated that vaccination with Hib conjugate vaccine leads to decreases in oropharyngeal colonization among both vaccinated and unvaccinated children (9–11); the prevalence of Hib carriage has decreased among preschool-aged children from 2%–7% in the prevaccine era to <1% in the vaccine era (9,12).
Several Hib-containing vaccines have been licensed since the initial Advisory Committee on Immunization Practices (ACIP) recommendations on prevention and control of Hib disease published in 1993 (13); subsequent publications have provided additional data and updated recommendations for these vaccines (14–17). This report summarizes previously published ACIP recommendations on prevention and control of Hib disease in immunocompetent and high-risk populations (14–18); it does not contain new recommendations and is intended as a resource for clinicians, public health officials, vaccination providers, and immunization program personnel. In addition, this report summarizes current information on Hib epidemiology in the United States and describes Hib vaccines licensed for use in the United States. Guidelines for antimicrobial chemoprophylaxis of contacts of persons with Hib disease also are provided.
Methods
ACIP's Meningococcal and Haemophilus influenzae type b Work Group* comprises a diverse group of health-care providers and public health officials. The Work Group includes professionals from academic medicine (pediatrics, family medicine, internal medicine, and infectious disease specialists), federal and state public health professionals, and representatives of professional medical organizations.
Published Hib vaccine recommendations were the primary sources of data used by the Work Group in summarizing recommendations for the prevention and control of Hib disease, including the evidence-based 2013 Infectious Diseases Society of America clinical practice guideline for vaccination of the immunocompromised host (17–23). Surveillance data came from the Active Bacterial Core surveillance (ABCs) system and the National Notifiable Diseases Surveillance System (NNDSS) (24).
Data on the immunogenicity and safety of current licensed and available Hib vaccines were summarized on the basis of findings from a literature search of PubMed and Web of Science databases that was completed on April 2, 2012. A nonsystematic review was conducted for studies on safety, effectiveness, and immunogenicity of the current Hib vaccines published from the time of vaccine licensure through March 2012. Because MenHibRix was licensed in June 2012, studies published before licensure also were reviewed. The literature search included clinical trials, randomized controlled trials, controlled clinical trials, evaluation studies, and comparative studies conducted worldwide and published in English. The Vaccine Adverse Events Reporting System (VAERS) (available at http://www.vaers.hhs.gov) also was searched for postlicensure safety data for the currently licensed and available Hib vaccines.
During December 2012–February 2013, the Work Group held one teleconference meeting and the members communicated with each other via e-mail messages to review current recommendations and to consider potential revisions to the statement. A summary of data reviewed, Work Group discussions, and the current description of Hib epidemiology was presented at the ACIP's February 2013 meeting. On February 20, 2013, ACIP members approved the Hib Vaccine Recommendations Statement. Modifications were made to the ACIP statement during the subsequent review process at CDC to update and clarify wording in the report.
Background
H. influenzae is a species of bacteria that has encapsulated (typeable) or unencapsulated (nontypeable) strains. Encapsulated strains express one of six antigenically distinct capsular polysaccharides (types a, b, c, d, e, or f). Encapsulated H. influenzae nontype b strains, particularly type a, can cause invasive disease similar to Hib disease (25,26). Nontypeable strains also can cause invasive disease but more commonly cause mucosal infections such as otitis media, conjunctivitis, and sinusitis. Hib vaccines only protect against H. influenzae type b strains; no vaccines against nontype b or nontypeable strains currently are available. H. influenzae colonizes the upper respiratory tract of humans and is transmitted person-to-person by inhalation of respiratory droplets or by direct contact with respiratory tract secretions.
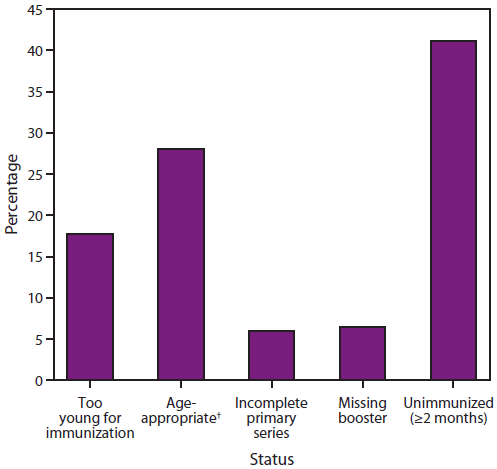
The majority of Hib disease in the United States occurs among unimmunized and underimmunized infants and children (those who have an incomplete primary series or are lacking a booster dose) and among infants too young to have completed the primary immunization series (27) (Figure 3). Although rare, Hib disease after full vaccination with the primary series and booster dose does occur; among Hib case-patients aged <5 years with age-appropriate vaccine status reported during 2002–2012 in the United States, 16% had completed the primary Hib series, and 43% had completed the full Hib series (Figure 3). Hib disease is uncommon in adults and in children aged >5 years. Additional information about H. influenzae disease is available at http://www.cdc.gov/hi-disease.
Persons with certain immunocompromising conditions are considered at increased risk for invasive Hib disease; these conditions might include:
- functional or anatomic asplenia,
- HIV infection,
- immunoglobulin deficiency including immunoglobulin G2 subclass deficiency,
- early component complement deficiency,
- receipt of a hematopoietic stem cell transplant, or
- receipt of chemotherapy or radiation therapy for malignant neoplasms.
Children who develop Hib disease despite appropriate vaccination should be evaluated for an immunological deficiency that predisposes them to Hib disease (28).
Historically, American Indian/Alaska Native (AI/AN) populations have had higher rates of Hib disease and colonization than the general U.S. population, with a peak in disease at a younger age (4–6 months) than among other U.S. infant populations (6–7 months) (29–31). Before introduction of vaccine in 1985, rates among AN children were five times higher than rates among non-AN children in Alaska (4). Although rates of Hib disease among AI/AN children have decreased in the postvaccine era, they remain higher than among non-AI/AN children. During 1998–2009, the average annual incidence of Hib disease in children aged <5 years in the United States was 8–10 times higher among AI/AN children (1.3/100,000) than it was among white (0.16/100,000) and black (0.12/100,000) children, respectively (27).
Development of Hib Vaccines
The first Hib vaccine licensed for use in the United States in 1985 was a monovalent vaccine consisting of purified polyribosylribitol phosphate (PRP) capsular material from type b strains. Although the vaccine was highly effective in trials in Finland among children aged ≥18 months, postmarketing effectiveness studies in the United States demonstrated variable effectiveness (-69%–88%) (32). PRP vaccines were ineffective in children aged <18 months because of the T lymphocyte-independent nature of the immune response to PRP polysaccharide (13). Conjugation of the PRP polysaccharide with protein carriers that contain T-lymphocyte epitopes confers T-lymphocyte-dependent characteristics to the vaccine. This conjugation enhances the immunologic response to the PRP antigen, particularly in young infants, and results in immunologic memory (e.g., anamnestic response) (33). Studies have suggested that long-term protection from invasive Hib disease is correlated with the presence of anti-PRP levels ≥0.15 µg/ml in unvaccinated children and anti-PRP levels ≥1.0 µg/ml in vaccinated children (34,35).
By 1989, three monovalent Hib conjugate vaccines were licensed for use among children aged ≥15 months (29). In late 1990, two of these conjugate vaccines were licensed for use among infants (36,37). Since 1990, additional Hib vaccines from numerous manufacturers have been licensed and are currently used in the United States, including monovalent Hib conjugate vaccines and combination vaccines that contain a Hib conjugate vaccine. No polysaccharide Hib vaccines are used currently in the United States.
Current Licensed and Available Hib Monovalent Conjugate Vaccines
As of January 1, 2014, three monovalent PRP polysaccharide-protein conjugate vaccines had been licensed by the Food and Drug Administration (FDA) and were available in the United States: PRP-OMP (PedvaxHIB, Merck and Co., Inc., Whitehouse Station, New Jersey), PRP-T (ActHIB, Sanofi Pasteur, Inc., Swiftwater, Pennsylvania), and PRP-T (Hiberix, GlaxoSmithKline, Research Triangle Park, North Carolina) (38) (Table 1).
In December 1990, PRP-OMP (PedvaxHIB) was licensed by FDA as a 2-dose primary series for infants at ages 2 and 4 months, with a booster dose (dose 3) at age 12 months (39). PRP-OMP contains purified PRP conjugated with an outer membrane protein complex (OMPC) of the B11 strain of Neisseria meningitidis serogroup B. Further information is available in the package insert at http://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM253652.pdf.
In March 1993, PRP-T (ActHIB) was licensed by FDA as a 3-dose primary series for infants at ages 2, 4, and 6 months, with a booster dose (dose 4) at age 15 months (40). This vaccine contains purified PRP conjugated with tetanus toxoid. Further information is available in the package insert at http://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM109841.pdf.
In August 2009, PRP-T (Hiberix) was licensed by FDA for use as the booster dose (which will be dose 3 or 4, depending on vaccine type used for primary series) of the Hib vaccine series for children aged 15 months through 4 years who have received a Hib primary series (16). This vaccine contains purified PRP conjugated with tetanus toxoid. Further information is available in the package insert at http://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM179530.pdf.
Current Licensed and Available Hib Combination Conjugate Vaccines
As of January 1, 2014, three combination vaccines that contain an H. influenzae type b conjugate vaccine had been licensed by FDA and were available in the United States: PRP-OMP/HepB (Comvax, Merck and Co., Inc., Whitehouse Station, New Jersey), DTaP-IPV/PRP-T (Pentacel, Sanofi Pasteur, Inc., Swiftwater, Pennsylvania), and MenCY/PRP-T (MenHibRix, GlaxoSmithKline, Inc., Rixensart, Belgium) (38) (Table 1).
In October 1996, PRP-OMP/HepB (Comvax) was licensed by FDA for vaccination against invasive Hib disease and hepatitis B infection in infants at ages 2, 4, and 12 through 15 months (14). This vaccine includes the antigenic components used in PedvaxHIB (PRP-OMP) and Recombivax HB (hepatitis B surface antigen). Further information is available in the package insert at http://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM109869.pdf.
In June 2008, DTaP/IPV/PRP-T (Pentacel) was licensed by FDA for vaccination against invasive Hib disease, diphtheria, tetanus, pertussis, and poliomyelitis in infants at ages 2, 4, 6, and 15 through 18 months (15). It is not indicated for the DTaP/IPV booster dose at age 4 through 6 years. The vaccine includes the antigenic components used in ActHIB (PRP-T) and Poliovax. Further information is available in the package insert at http://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM109810.pdf.
In June 2012, MenCY/PRP-T (MenHibRix) was licensed by FDA for vaccination against invasive Hib disease and N. meningitidis serogroups C and Y disease in infants at ages 2, 4, 6, and 12 through 15 months (17). Infants at increased risk for meningococcal disease† should be vaccinated with a 4-dose series of MenCY/PRP-T. Routine meningococcal vaccination is recommended only for infants who are at increased risk for meningococcal disease. However, MenCY/PRP-T may be used in any infant for routine vaccination against Hib. Further recommendations for use of the MenCY component of MenCY/PRP-T have been published previously (17). Further vaccine information is available in the package insert at http://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM308577.pdf.
Immunogenicity of Current Licensed and Available Hib Vaccines
Protective antibody levels are detected for both PedvaxHib and ActHib after a primary series (41–43). However, the vaccines differ in the timing of antibody response. PedvaxHib produces a substantial antibody response after the first dose with an additional boost in geometric mean antibody concentration after the second or third dose (41,42,44–46). Therefore, PedvaxHib is licensed as a 2-dose primary series. PedvaxHib effectiveness was 93%–100% in Navajo infants vaccinated with a 2-dose series (13,41,47).
Geometric mean antibody concentrations remain at or below 1.0 µg/ml after the first and second dose of ActHIB, but a protective antibody response is seen after the third dose (41,42,45,48,49). Effectiveness studies for ActHIB were terminated early in the United States with licensure of the first Hib conjugate vaccine; no cases of invasive Hib disease were reported among vaccinees at the time of study termination (13,47). A prospective controlled trial of PRP-T among 56,000 subjects in the United Kingdom found an effectiveness of 95% (95% confidence interval [CI] = 74%–100%) (47).
Antibody levels decline after completion of the primary series with PRP-T and PRP-OMP vaccines and a booster dose at age 12–15 months is necessary to maintain protective antibody levels. Booster doses of PedvaxHib, ActHib, and Hiberix at age 12–15 months provide levels of antibody that are protective against invasive Hib disease (16,44,46,48,50,51).
Protective antibody responses comparable to those detected after receipt of separately administered PedVaxHIB and Recombivax HB vaccines are seen after the second primary dose and booster dose of Comvax vaccine (14,52). Pentacel and MenHibRix induce protective antibody responses that are noninferior to separately administered PRP-T vaccines after the third primary dose and booster dose (53–59).
No clinically significant immune interference has been observed with any of the available monovalent or combination Hib vaccines and concomitant administration of other routine childhood vaccines (51,52,60–67; ACIP, unpublished data, 2009).
Safety of Current Licensed and Available Hib Vaccines
In prelicensure trials, adverse reactions to PedvaxHib, ActHib, and Hiberix were uncommon, usually mild, and generally resolved within 12–24 hours (16,41,43,46,49,50). Rates of adverse reactions to Comvax, Pentacel, and MenHibRix were similar to those seen with separately administered vaccines (14,52–54,68).
Postmarketing surveillance for adverse events following receipt of Hib vaccines has been conducted primarily by two systems in the United States: VAERS and the Vaccine Safety Datalink (VSD). VAERS is a national passive surveillance system operated jointly by CDC and FDA that receives reports of adverse events following vaccination from health-care personnel, manufacturers, vaccine recipients, and others (69). VAERS can generate, but not test, vaccine safety hypotheses and is subject to several limitations, including reporting biases and inconsistent data quality (69). VSD is a collaboration between CDC and nine integrated health-care organizations that conducts population-based vaccination safety studies to assess hypotheses that arise from review of medical literature, reports to VAERS, changes in immunization schedules, or introduction of new vaccines (70).
Safety Data Reported to VAERS
During January 1, 1990–May 31, 2013, VAERS received 29,047 reports involving receipt of Hib vaccines (PedvaxHIB, ActHIB, Hiberix, Comvax, and Pentacel) in the United States; 26,375 (91%) reports involved children aged <2 years. Hib vaccines were administered concurrently with one or more other vaccines in 95% of case reports. The median time from vaccination to onset of an adverse event was 1 day. The most frequently reported adverse events were fever (31%), crying (11%), injection site erythema (11%), irritability (10%), and rash (9%).
Among all Hib vaccines reports, approximately 17% were coded as serious as defined in the Code of Federal Regulations (71) (i.e., report contained information that the event led to death, life-threatening illness, hospitalization, prolongation of hospitalization, or permanent disability). Among the 5,062 reports coded as serious, the most frequent adverse events were fever (37%), vomiting (21%), convulsion (20%), irritability (17%), and intussusception (11%). In 97% of the intussusception reports, rotavirus vaccine was administered concomitantly and might have prompted reporting of this adverse event.
VAERS received reports of 878 deaths following Hib containing vaccines that occurred during January 1, 1990–May 31, 2013. An autopsy report or other medical records was available for 620 (71%) of these deaths, among which the most frequent cause of death was sudden infant death syndrome (52%). Other causes of death included respiratory (9%), cardiovascular (5%), infectious (5%), neurologic (3%), and gastrointestinal (2%) conditions. In 14% of reports, the cause was undetermined, and in 11% of reports, various other causes were reported (e.g., asphyxia and blunt force trauma).
The reporting frequencies for Hib containing vaccines are similar to what has been observed with other recommended childhood vaccines. No unusual or unexpected safety patterns were observed in VAERS data for any Hib vaccines.
Population-Based Safety Findings
No postlicensure safety studies of monovalent Hib vaccines were identified by the literature review. However, the VSD conducted an observational study of the combination Hib vaccine, DTaP-IPV-Hib (Pentacel), for the period September 2008–January 2011 (55). Compared with children who received DTaP-containing control vaccine (i.e., without Hib), children aged 1–2 years who received DTaP-IPV-Hib vaccine had an elevated risk for fever (RR = 1.83; 95% CI = 1.34–2.50). DTaP-IPV-Hib vaccine was not associated with any other medically attended adverse health event.
An independent postmarketing safety evaluation of Hib-HepB (Comvax) was conducted by a managed care organization in Seattle, Washington, for the period July 1997–December 2000 (72). Using ICD-9 codes, the retrospective cohort study evaluated adverse events reported 1–30 days following administration of Hib-HepB, compared with rates of adverse events among two control groups (historical control group and self-comparison group). A total of 27,802 vaccine doses were administered during the study period with 111,129 diagnoses recorded within 0–30 days following administration of Comvax in any health-care setting. There were 127 separate adverse event codes with significant elevated relative risks and 66 codes with significantly decreased relative risks (p<0.5). On medical record review, there was no consistent pattern to respiratory or gastrointestinal illnesses; fever findings appeared to be explained by changes in data collection or by concomitant vaccination with measles, mumps, and rubella virus vaccine. Two deaths occurred within the study period, both of which were considered unrelated to vaccination. No consistent association was identified between serious adverse events and vaccination with Hib-HepB, and the vaccine had a favorable safety profile.
Recommendations for Hib Vaccine Use
Recommendations for Routine Vaccination
ACIP recommends routine administration of a conjugate Hib vaccine series (monovalent vaccine [PedvaxHib (PRP-OMP) or ActHib (PRP-T)] or Hib vaccine in combination with HepB [Comvax], DTaP/IPV [Pentacel], or MenCY [MenHibRix]) beginning at age 2 months. Infants aged 2–6 months should receive a 3-dose series of Hib PRP-T as ActHib, Pentacel, or MenHibRix or a 2-dose series of Hib PRP-OMP as PedvaxHib or Comvax (Table 1). The first dose can be administered as early as age 6 weeks. A booster dose (which will be dose 3 or 4 depending on vaccine type used in primary series) of any licensed conjugate Hib vaccine (monovalent vaccine [PedvaxHib (PRP-OMP), ActHib (PRP-T), or Hiberix (PRP-T)] or Hib vaccine in combination with HepB [Comvax] or DTaP/IPV [Pentacel] or MenCY [MenHibRix]) is recommended at age 12 through 15 months and at least 8 weeks after the most recent Hib vaccination (Table 1).
Hib vaccine has been found to be immunogenic in patients with immunocompromising conditions although immunogenicity varies with the degree of immunocompetence (13,73–84). Patients at increased risk for invasive Hib disease who are vaccinated (have received a Hib primary series and a booster dose at age ≥12 months) do not need further routine immunization, except in certain situations (Table 2).
Guidance for Hib Vaccine Use
Guidance for Routine Vaccination
Doses for either primary series (2-dose or 3-dose) should be administered 8 weeks apart; however, if necessary, an interval of 4 weeks between doses is acceptable. If a PRP-OMP vaccine (PedvaxHIB or Comvax) is administered for both doses in the primary series, a third primary dose is not indicated. If a PRP-OMP vaccine (PedvaxHib or Comvax) is not administered for both doses in the primary series or there is uncertainty about which products were administered previously, a third primary series dose of a Hib conjugate vaccine is needed to complete the primary series. Any monovalent or combination Hib conjugate vaccine is acceptable for the booster dose (dose 3 or 4 depending on vaccine type used in primary series), regardless of the product used for the primary series. Hiberix should be used only for the booster dose (dose 3 or 4, depending on the vaccine type used for primary series) in children aged 12 months through 4 years who have received at least 1 dose of Hib vaccine previously.
Guidance for Catch-up Schedules
If the first vaccination is delayed by >1 month, the recommended catch-up schedule (available at http://www.cdc.gov/vaccines/schedules/hcp/child-adolescent.html) should be followed.
- For unvaccinated infants receiving the first dose at age <7 months, 2 doses of PRP-OMP or 3 doses of PRP-T vaccine should be administered with a minimum of a 4-week interval between doses. A booster dose (dose 3 or 4 depending on vaccine type used for primary series) at age 12 through 15 months is necessary only if 2 or 3 primary doses (depending on vaccine type used) were administered before age 12 months.
- For unvaccinated infants receiving the first dose at age 7 through 11 months, a second dose should be administered at least 4 weeks later (regardless of Hib conjugate vaccine [PRP-T or PRP-OMP] used for first dose). A third (and final) dose should be administered at age 12 through 15 months or 8 weeks after the second dose, whichever date is later.
- For unvaccinated children receiving the first dose at age 12 through 14 months, a second dose of any monovalent or combination Hib conjugate vaccine should be administered 8 weeks after the first dose. A third dose is not necessary.
- For unvaccinated children receiving the first dose at age 15 through 59 months, no further doses of any monovalent or combination Hib conjugate vaccine are indicated.
- Previously unvaccinated children aged ≥60 months who are not considered high-risk generally are immune to Hib disease and do not require catch-up vaccination.
The recommended catch-up schedule should be followed for children aged <12 months who are at increased risk for Hib disease and have delayed or no Hib vaccination. Catch-up guidance for children aged 12 through 59 months who are at increased risk for Hib disease and who have delayed or no Hib vaccination is described below (see "High-risk groups"; Table 2).
Guidance for Vaccinating Special Populations
American Indians/Alaska Natives
Hib meningitis incidence peaks at a younger age (4–6 months) among AI/AN infants than among other U.S. infant populations (6–7 months) (29–31). Vaccination with a 2 dose primary series of a Hib vaccine that contains PRP-OMP (PedvaxHIB or Comvax) is preferred for AI/AN infants to provide early protection because these vaccines produce a protective antibody response after the first dose (41–43,51,52,85). If the first vaccination dose is delayed by >1 month, the recommended catch-up schedule (available at http://www.cdc.gov/vaccines/schedules/hcp/child-adolescent.html) should be followed. A booster dose (dose 3) of Hib vaccine is recommended at age 12 through 15 months; for the booster dose, there is no preferred vaccine formulation (i.e., any licensed Hib conjugate vaccine is acceptable). The importance of this early protection was demonstrated in Alaska (30,86). During July 1991–January 1996, a PRP-OMP vaccine was used statewide in Alaska and a >90% decrease in Hib disease rates occurred among AN and nonnative children (30,86). During 1996–1997, after the statewide vaccine was changed to a combination vaccine that included a non-OMP Hib component, Hib incidence increased significantly (19.8 to 91.1 cases/100,000 children aged <5 years, p<0.001) among AN children while remaining unchanged among nonnative children (30,86). Disease reappearance seemed to be attributable to the use of a Hib vaccine that did not achieve early protective antibody concentrations in children who had ongoing exposure to Hib via oropharyngeal colonization among close contacts. After returning to the use of PRP-OMP containing vaccines in Alaska, the incidence of Hib disease in AN children decreased to rates of fewer than six cases per 100,00 children aged <5 years (30,86).
Children Aged <24 Months with Invasive Hib Disease
Children aged <24 months who develop invasive Hib disease can remain at risk for developing a second episode because natural infection in this age group does not reliably result in development of protective antibody levels. These children should be considered unvaccinated regardless of previous Hib vaccination and should receive Hib vaccine doses according to the age-appropriate schedule for unimmunized children (28,87–89). Children aged <24 months who develop invasive Hib disease should receive primary vaccination or re-vaccination with a second primary series beginning 4 weeks after onset of disease.
Preterm Infants
Medically stable preterm infants§ should be vaccinated beginning at age 2 months according to the schedule recommended for other infants, on the basis of chronological age.
High Risk Groups
Persons considered at increased risk for invasive Hib disease include those with functional or anatomic asplenia, HIV infection, immunoglobulin deficiency (including immunoglobulin G2 subclass deficiency), or early component complement deficiency, recipients of a hematopoietic stem cell transplant, and those receiving chemotherapy or radiation therapy for malignant neoplasms. A single dose of any licensed Hib conjugate vaccine should be administered to unimmunized older children, adolescents, and adults who are asplenic or who are scheduled for an elective splenectomy. Some experts suggest administering a dose prior to elective splenectomy regardless of prior vaccination history (22). On the basis of limited data on the timing of Hib vaccination before splenectomy, experts suggest vaccination at least 14 days before the procedure (18,19,23) (Table 2).
Unimmunized children aged ≥60 months who have HIV infection should receive 1 dose of Hib vaccine. Whether HIV-infected children who have received a full 3 or 4 dose vaccine series (depending on the vaccine type used for the primary series) will benefit from additional Hib doses is unknown. Because the incidence of Hib infections among HIV-infected adults is low, Hib vaccine is not recommended for adults with HIV infection (21,23) (Table 2).
Children aged 12–59 months who are at increased risk for Hib disease (persons with asplenia, HIV infection, immunoglobulin deficiency, early component complement deficiency, or chemotherapy or radiation therapy recipients) and who received no doses or only 1 dose of Hib conjugate vaccine before age 12 months should receive 2 additional doses of vaccine 8 weeks apart; children who received 2 or more doses of Hib conjugate vaccine before age 12 months should receive 1 additional dose, at least 8 weeks after the last dose (Table 2).
Hib vaccination during chemotherapy or radiation therapy should be avoided because of possible suboptimal antibody response. Patients vaccinated within 14 days of starting immunosuppressive therapy or while receiving immunosuppressive therapy should be considered unimmunized, and doses should be repeated beginning at least 3 months following completion of chemotherapy. Patients who were vaccinated more than 14 days before chemotherapy do not require revaccination, with the exception of recipients of a hematopoietic stem cell transplant who should be revaccinated with a 3-dose regimen 6–12 months after successful transplant, regardless of vaccination history (80); at least 4 weeks should separate doses (Table 2).
Guidance for Vaccine Administration
Hib vaccines are administered intramuscularly in individual doses of 0.5 mL. Adverse events occurring after administration of any vaccine should be reported to VAERS. Reports can be submitted to VAERS online, by facsimile, or by mail. More information about VAERS is available by calling 1-800-822-7967 (toll-free) or online at http://vaers.hhs.gov.
Interchangeability of Vaccine Product
Studies have demonstrated that any combination of licensed monovalent Hib conjugate vaccines for the primary and booster doses provide comparable or higher antibody levels than with the same monovalent product (33,44–46,51,91–93). Therefore, licensed monovalent Hib conjugate vaccines are considered interchangeable for the primary as well as the booster doses (dose 3 or 4, depending on vaccine type used for primary series) (18,94). Data on the interchangeability of combination vaccines with other combination vaccines or with monovalent vaccines are limited (52,63). Whenever feasible, the same combination vaccine should be used for the subsequent doses; however, if a different brand is administered, the dose should be considered valid and need not be repeated.
Precautions and Contraindications
Adverse reactions to Hib-containing monovalent vaccines are uncommon, usually mild, and generally resolve within 12–24 hours (41–43,49). Rates of adverse reactions to Hib combination vaccines are similar to those observed with separately administered vaccines (14,33,52–54). More complete information about adverse reactions to a specific vaccine is available in the package insert for each vaccine and from CDC at http://www.cdc.gov/vaccines/vac-gen/side-effects.htm.
Vaccination with a Hib-containing vaccine is contraindicated in infants aged <6 weeks. Vaccination with a Hib-containing vaccine is contraindicated among persons known to have a severe allergic reaction to any component of the vaccine. The tip caps of the Hiberix prefilled syringes might contain natural rubber latex, and the vial stoppers for Comvax, ActHib, and PedvaxHIB contain natural rubber latex, which might cause allergic reactions in persons who are latex-sensitive. Therefore, vaccination with these vaccines is contraindicated for persons known to have a severe allergic reaction to dry natural rubber latex (48,50–52). The vial stoppers for Pentacel and MenHibRix do not contain latex (63,95). Vaccination with Comvax is contraindicated in patients with a hypersensitivity to yeast (52).
As with all pertussis-containing vaccines, benefits and risk should be considered before administering Pentacel to persons with a history of fever ≥40.5oC, hypotonic-hyporesponsive episode, persistent inconsolable crying lasting ≥3 hours within 48 hours after receipt of a pertussis-containing vaccine, or seizures within 3 days after receiving a pertussis-containing vaccine (63).
Hib monovalent and combination conjugate vaccines are inactivated vaccines and may be administered to persons with immunocompromising conditions. However, immunologic response to the vaccine might be suboptimal (18).
Guidance for Chemoprophylaxis
Secondary cases of Hib disease (illness occurring within 60 days of contact with a patient) occur but are rare. Secondary attack rates are higher among household contacts aged <48 months (2.1%), especially those aged <12 months (6%) and <24 months (3%) (29). Data are conflicting on the risk for secondary illness among child care contacts, but it is thought to be lower than among household contacts (29). Rifampin is recommended for chemoprophylaxis because it achieves high concentrations in respiratory secretions and eradicates nasopharyngeal carriage in >95% of carriers (96–99). There are no guidelines for control measures around cases of invasive nontype b H. influenzae disease. Chemoprophylaxis is not recommended for contacts of persons with invasive disease caused by nontype b H. influenzae because cases of secondary transmission of disease have not been documented (100,101).
Index Patients with Invasive Hib Disease
Index patients who are treated with an antibiotic other than cefotaxime or ceftriaxone and are aged <2 years should receive rifampin prior to hospital discharge (22). Because cefotaxime and ceftriaxone eradicate Hib colonization, prophylaxis is not needed for patients treated with either of these antimicrobials.
Household Contacts
Rifampin chemoprophylaxis is recommended for index patients (unless treated with cefotaxime or ceftriaxone) and all household contacts in households with members aged <4 years who are not fully vaccinated or members aged <18 years who are immunocompromised, regardless of their vaccination status (22).
Child Care Contacts
Rifampin chemoprophylaxis is recommended in child care settings when two or more cases of invasive Hib disease have occurred within 60 days and unimmunized or underimmunized children attend the facility (22). When prophylaxis is indicated, it should be prescribed for all attendees, regardless of age or vaccine status, and for child care providers.
Conclusion
Hib disease was once a leading cause of bacterial meningitis among U.S. children aged <5 years. As a result of the introduction of Hib vaccines in the United States and sustained high vaccine coverage, Hib disease is now rare, with rates below the Healthy People 2020 objective. However, the risk for invasive Hib disease continues among unimmunized and underimmunized children, highlighting the importance of full vaccination with the primary series and booster doses. Although Hib disease is uncommon, continued H. influenzae surveillance with complete serotyping data is necessary so that all Hib cases are identified and appropriate chemoprophylaxis measures can be taken.
References
- Wenger JD, Hightower AW, Facklam RR, Gaventa S, Broome CV. Bacterial meningitis in the United States, 1986: report of a multistate surveillance study. J Infect Dis 1990;162:1316–23.
- Broome CV. Epidemiology of Haemophilus influenzae type b infections in the United States. Pediatr Infect Dis J 1987;6:779–82.
- Adams WG, Deaver KA, Cochi SL, et al. Decline of Childhood Haemophilus influenzae type b (Hib) Disease in the Hib Vaccine Era. JAMA 1993;269:221–6.
- Bisgard KM, Kao A, Leake J, Strebel PM, Perkins BA, Wharton M. Haemophilus influenzae invasive disease in the United States, 1994–1995: near disappearance of a vaccine-preventable childhood disease. Emerg Infect Dis 1998;4:229–37.
- CDC. Progress toward elimination of Haemophilus influenzae type b disease among infants and children—United States, 1987–1995. MMWR 1996;45:901–6.
- CDC. Progress toward elimination of Haemophilus influenzae type b disease among infants and children—United States, 1987–1997. MMWR 1998;47:993–8.
- CDC. Progress toward elimination of Haemophilus influenzae type b disease among infants and children—United States, 1998–2000. MMWR 2002;51:234–7.
- US Department of Health and Human Services. Healthy people 2020 topics and objectives. Washington, DC: US Department of Health and Human Services; 2013. Available at http://www.healthypeople.gov/2020/topicsobjectives2020/objectiveslist.aspx?topicid=23.
- Mohle-Boetani J, Ajello G, Breneman E, et al. Carriage of Haemophilus influenzae type b in children after widespread vaccination with conjugate Haemophilus influenzae type b vaccines. Pediatr Infect Dis J 1993;12:589–93.
- Takala A, Eskola J, Leinonen M, et al. Reduction of oropharyngeal carriage of Haemophilus influenzae type b (Hib) in children immunized with an Hib conjugate vaccine. J Infect Dis 1991;164:982–6.
- Barbour M, Phil D. Conjugate Vaccines and the Carriage of Haemophilus influenzae type b. Emerg Infect Dis 1996;2:176–82.
- Lowther SA, Shinoda N, Juni BA, et al. Haemophilus influenzae type b infection, vaccination, and H. influenzae carriage among children in Minnesota, 2008–2009. Epidemiol Infect 2012;140:566–74.
- CDC. Recommendations for use of Haemophilus b conjugate vaccines and a combined diphtheria, tetanus, pertussis, and Haemophilus b vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 1993;42(No. RR-13).
- CDC. FDA approval for infants of a Haemophilus influenzae type b conjugate and hepatitis B (recombinant) combined vaccine. MMWR 1997;46:107–9.
- CDC. Licensure of a diphtheria and tetanus toxoids and acellular pertussis adsorbed, inactivated poliovirus, and Haemophilus b conjugate vaccine and guidance for use in infants and children. MMWR 2008;57:1079–80.
- CDC. Licensure of a Haemophilus influenzae type b (Hib) vaccine (Hiberix) and updated recommendations for use of Hib vaccine. MMWR 2009;58:1008–9.
- CDC. Infant meningococcal vaccination: Advisory Committee on Immunization Practices (ACIP) recommendations and rationale. MMWR 2013;62:52–4.
- CDC. General recommendations on immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2011;60(No. RR-2).
- CDC. Recommendations of the Advisory Committee on Immunization Practices (ACIP): use of vaccines and immune globulins in persons with altered immunocompetence. MMWR 1993;42(No. RR-4).
- CDC. Guidelines for the prevention and treatment of opportunistic infections among HIV-exposed and HIV-infected children: recommendations from CDC, the National Institutes of Health, the HIV Medicine Association of the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the American Academy of Pediatrics. MMWR 2009;58(No. RR-11).
- CDC. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR 2009;58(No. RR-4).
- American Academy of Pediatrics. Haemophilus influenzae infections. In: Pickering L, Baker C, Kimberlin D, Long S, eds. Red book: 2012 report of the Committee on Infectious Diseases. Elk Grove Village, IL: American Academy of Pediatrics; 2012:345–52.
- Rubin LG, Levin MJ, Ljungman P, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompormised host. Clin Infect Dis 2013;[Epub ahead of print] doi: 10.1093/cid/cit684.
- Schuchat A, Hilger T, Zell E, et al. Active bacterial core surveillance of the emerging infections program network. Emerg Infect Dis 2001;7:92–9.
- Bruce MG, Deeks SL, Zulz T, et al. Epidemiology of Haemophilus influenzae serotype a, North American Arctic, 2000–2005. Emerg Infect Dis 2008;14:48–55.
- Adderson EE, Byington L, Spencer L, et al. Invasive serotype a Haemophilus influenzae infections with a virulence genotype resembling Haemophilus influenzae Type b: emerging pathogen in the vaccine era? Pediatrics 2001;108:e18.
- Briere EJM, Jackson M, Shah SG, et al. Haemophilus influenzae type b disease and vaccine booster dose deferral, United States, 1998–2009. Pediatrics 2012;130:414–20.
- Heath P, Booy R, Griffiths H, et al. Clinical and immunological risk factors associated with Haemophilus influenzae type b conjugate vaccine failure in childhood. Clin Infect Dis 2000;31:973–80.
- Wenger JD, Ward JI. Haemophilus influenzae vaccine. In: Plotkin SA, Orenstein WA, Offit PA, eds. Vaccines. 4th ed: W.B. Saunders Company; 2003:229–68.
- Singleton R, Hammitt L, Hennessy T, et al. The Alaska Haemophilus influenzae type b experience: lessons in controlling a vaccine-preventable disease. Pediatrics 2006;118:e421–9.
- Coulehan J, Michaels R, Hallowell C. Epidemiology of Haemophilus influenzae type B disease among Navajo Indians. Public Health Rep 1984;99:404–9.
- Ward JI, Broome CV, Harrison LH, Shinefield H, Black S. Haemophilus influenzae type b vaccines: Lessons for the Future. Pediatrics 1988;81:886–93.
- Murphy TV. Haemophilus influenzae vaccines: 1997. In: Aronoff SC. Advances in pediatric infectious diseases. San Diego, CA: Mosby-Year Book, Inc.; 1998.
- Shapiro ED, Ward JI. The epidemiology and prevention of disease caused by Haemophilus influenzae type b. Epidemiol Rev 1991;13:113–42.
- Kayhty H, Peltola H, Karanko V, Makela PH. The protective level of serum antibodies to the capsular polysaccharide of Haemophilus influenzae Type b. J Infect Dis 1983;147:1100.
- Black SB, Shinefield HR, Fireman B, Hiatt R, Polen M, Vittinghoff E. Efficacy in infancy of oligosaccharide conjugate Haemophilus influenzae type b (HbOC) vaccine in a United States population of 61,080 children. Pediatr Infect Dis J 1991;10:97–104.
- Santosham M, Wolff M, Reid R, Hohenboken M, Bateman M. The efficacy in Navajo infants of a conjugate vaccine consisting of Haemophilus influenzae type b polysaccharide and Neisseria meningitidis outer-membrane protein complex. N Engl J Med 1991;324:1767–72.
- Food and Drug Administration. Complete list of vaccines licensed for immunization and distribution in the US. Silver Springs, MD: Food and Drug Administration; 2012. Available at http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm093833.htm.
- CDC. Food and Drug Administration approval of use of a Haemophilus b conjugate vaccine for infants. MMWR 1990;39:925.
- CDC. FDA approval of use of a new Haemophilus b conjugate vaccine and a combined diphtheria-tetanus-pertussis and Haemophilus b conjugate vaccine for infants and children. MMWR 1993;42:296–8.
- Decker MD, Edwards KM, Bradley R, Palmer P. Comparative trial in infants of four conjugate Haemophilus influenzae type b vaccines. J Pediatr 1992;120:184–9.
- Decker MD, Edwards KM. Haemophilus influenzae type b vaccines: history, choice, and comparisons. Pediatr Infect Dis J 1998;17:S113–6.
- Fritzell B, Plotkin S. Efficacy and safety of a Haemophilus influenzae type b capsular polysaccharide-tetanus protein conjugate vaccine. J Pediatr 1992;121:355–62.
- Anderson E, Decker MD, Englund J, et al. Interchangeability of conjugated Haemophilus influenzae type b vaccines in infants. JAMA 1995;273:849–53.
- Bewley KM, Schwab JG, Ballanco GA, Daum RS. Interchangeability of Haemophilus influenzae type b vaccines in the primary series: evaluation of a two-dose mixed regimen. Pediatrics 1996;98:898–904.
- Greenberg DP, Feldman S. Vaccine interchangeability. Clin Pediatr (Phila) 2003;42:93–9.
- Heath P. Haemophilus influenzae type b conjugate vaccines: a review of efficacy data. Pediatr Infect Dis J 1998;17(Suppl):S117–22.
- Sanofi Pasteur. Haemophilus b conjugate vaccine (tetanus toxoid conjugate) ActHIB [Package insert]. Available at http://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM109841.pdf.
- Holmes SJ, Fritzell B, Guito KP, et al. Immunogenicity of Haemophilus influenzae type b polysaccharide-tetanus toxoid conjugate vaccine in infants. Am J Dis Child 1993;147:832–6.
- GlaxoSmithKline. HIBERIX (Haemophilus b conjugate vaccine [tetanus toxoid conjugate]) [Package insert]. Available at http://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM179530.pdf.
- Merck & Co., Inc. Liquid Pedvax HIB (Haemophilus b conjugate vaccine (meningococcal protein conjugate). [Package insert.] Available at http://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM253652.pdf.)
- Merck & Co., Inc. COMVAX (Haemophilus b conjugate [meningococcal protein conjugate] and hepatitis B (recombinant) vaccine) [Package insert]. Available at http://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM109869.pdf.
- Guerra FA, Blatter MM, Greenberg DP, Pichichero M, Noriega FR. Pentacel Study G. Safety and immunogenicity of a pentavalent vaccine compared with separate administration of licensed equivalent vaccines in US infants and toddlers and persistence of antibodies before a preschool booster dose: a randomized, clinical trial. Pediatrics 2009;123:301–12.
- Black S, Greenberg DP. A combined diphtheria, tetanus, five-component acellular pertussis, poliovirus, and Haemophilus influenzae type b vaccine. Expert Rev Vaccines 2005;4:793–805.
- Nelson J, Yu O, Dominguez-Islas C, et al. Adapting group sequential methods to observational postlicensure vaccine safety surveillance: results of a pentavalent combination DTaP-IPV-Hib vaccine safety study. Am J Epidemiol 2013;177:131–41.
- Marchant CD, Miller JM, Marshall GS, et al. Randomized trial to assess immunogenicity and safety of Haemophilus influenzae type b and Neisseria meningitidis serogroups C and Y-tetanus toxoid conjugate vaccine in infants. Pediatr Infect Dis J 2010;29:48–52.
- Marshall GS, Marchant CD, Blatter M, et al. Immune response and one-year antibody persistence after a fourth dose of a novel Haemophilus influenzae type b and Neisseria Meningitidis serogroups C and Y-tetanus toxoid conjugate vaccine (HibMenCY) at 12 to 15 months of age. Pediatr Infect Dis J 2010;29:469–71.
- Nolan T, Lambert S, Roberton D, et al. A novel combined Haemophilus influenzae type b-Neisseria meningitidis serogroups C and Y-tetanus-toxoid conjugate vaccine is immogenic and induces immune memory when co-administered with DTPa-HBV-IPV and conjugate pneumococcal vaccines in infants. Vaccine 2007;25:8487–99.
- Nolan T, Richmond P, Marshall H, et al. Immunogenicity and safety of an investigational combined Haemophilus influenzae type b-Neisseria meningitidis serogroups C and Y-tetanus toxoid conjugate vaccine. Pediatr Infect Dis J 2011;30:190–6.
- Usonis V, Bakasenas V. Does concomitant injection of a combined diphtheria-tetanus-acellular pertussis-hepatitis B virus–inactivated polio virus vaccine influence the reactogenicity and immunogenicity of commercial Haemophilus influenzae type b conjugate vaccines? Eur J Pediatr 1999;158:398–402.
- Reuman PD, Sawyer MH, Kuter BJ, et al. Safety and immunogenicity of concurrent administration of measles-mumps-rubella-varicella vaccine and PedvaxHIB(R) vaccines in healthy children twelve to eighteen months old. Pediatr Infect Dis J 1997;16:662–7.
- Schmitt HJ, Zepp F, Muschenborn S, et al. Immunogenicity and reactogenicity of a Haemophilus influenzae type b tetanus conjugate vaccine when administered separately or mixed with concomitant diphtheria-tetanus-toxoid and acellular pertussis vaccine for primary and for booster immunizations. Eur J Pediatr 1998;157:208–14.
- Sanofi Pasteur. Pentacel (Diphtheria and tetanus toxoids and acellular pertussis adsorbed, inactivated poliovirus and Haemophilus b conjugate (tetanus toxoid conjugate) vaccine suspension for intramuscular injection [Package insert]. Available at http://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM109810.pdf.
- Dhillon S, Keam SJ. DTaP-IPV/Hib vaccine (Pentacel). Paediatr Drugs 2008;10:405–16.
- Hesley TM, Reisinger KS, Sullivan BJ, et al. Concomitant administration of a bivalent Haemophilus influenzae type b-hepatitis B vaccine, measles-mumps-rubella vaccine and varicella vaccine: safety, tolerability and immunogenicity. Pediatr Infect Dis J 2004;23:240–5.
- Bernstein HH, Noriega F, Group MAPS. Immunogenicity and safety of a combined diphtheria, tetanus, 5-component acellular pertussis, inactivated poliomyelitis, Haemophilus type b conjugate vaccine when administered concurrently with a pneumococcal conjugate vaccine: a randomized, open-label, phase 3 study. Vaccine 2011;29:2212–21.
- Dennehy PH, Bertrand HR, Silas PE, Damaso S, Friedland LR, Abu-Elyazeed R. Coadministration of RIX4414 oral human rotavirus vaccine does not impact the immune response to antigens contained in routine infant vaccines in the United States. Pediatrics 2008;122:e1062–6.
- Rinderknecht S, Bryant KA, Nolan T, et al. The safety profile of Haemophilus influenzae type b-Neisseria meningitidis serogroups C and Y tetanus toxoid conjugate vacine (HibMenCY). Hum Vaccin Immunother 2012;8:304–11.
- Varricchio F, Iskander J, Destefano F, et al. Understanding vaccine safety information from the Vaccine Adverse Event Reporting System. Pediatr Infect Dis J 2004;23:287–94.
- Baggs J, Gee J, Lewis E, et al. The Vaccine Safety Datalink: a model for monitoring immunization safety. Pediatrics 2011;127:S45–53.
- Food and Drug Administration. 21 CFR Part 600.80. Postmarketing reporting of adverse experiences. Fed Regist 1997;62:52252–3.
- Davis RL, Black S, Shinefield H, et al. Post-marketing evaluation of the short term safety of COMVAX (R). Vaccine 2004;22:536–43.
- Ek T, Mellander L, Hahn-Zoric M, Abrahamsson J. Intensive treatment for childhood acute lymphoblastic leukemia reduces immune responses to diphtheria, tetanus, and Haemophilus influenzae type b. J Pediatr Hematol Oncol 2004;26:727–34.
- Daza P, Banda R, Misoya K, et al. The impact of routine infant immunization with Haemophilus influenzae type b conjugate vaccine in Malawi, a country with high Human Immunodeficiency Virus prevalence. Vaccine 2006;24:6232–9.
- Madhi SA. Immunogenicity and effectiveness of Haemophilus influenzae type b conjugate vaccine in HIV infected and uninfected African children. Vaccine 2005;23:5517–25.
- Madhi SA, Petersen K, Khoosal M, et al. Reduced effectiveness of Haemophilus influenzae type b conjugate vaccine in children with a high prevalence of human immunodeficiency virus type 1 infection. Pediatr Infect Dis J 2002;21:315–21.
- Parkkali T, Kayhty H, Ruutu T, Volin L, Eskola J, Ruutu P. A comparison of early and late vaccination with Haemophilus influenzae type b conjugate and pneumococcal polysaccharide vaccines after allogeneic BMT. Bone Marrow Transplant 1996;18:961–7.
- Guinan EC, Molrine D, Antin J, et al. Polysaccharide conjugate vaccine responses in bone marrow transplant patients. Transplantation 1994;57:677–84.
- Barra A, Cordonnier C, Preziosi M-P, et al. Immunogenicity of Haemophilus influenzae type b conjugate vaccine in allogenic bone marrow recipients. J Infect Dis 1992;166:1021–8.
- Avanzini MA, Carra AM, Maccario R, et al. Immunization with Haemophilus influenzae type b conjugate vaccine in children given bone marrow transplantation: comparison with healthy age-matched controls. J Clin Immunol 1998;1998:3.
- Newcomer W, Santosham M, Bengston S, Panny S, Dover G. Immunogenicity of Haemophilus influenzae type b polysaccharide and Neisseria meningitidis outer membrane protein complex conjugate vaccine in infants and children with sickle cell disease. Pediatr Infect Dis J 1993;12:1026–7.
- Jakacki R, Luery N, McVerry P, Lange B. Haemophilus influenzae diptheria protein conjugate immunization after therapy in splenectomized patients with Hodgkin Disease. Ann Intern Med 1990;112:143–4.
- Frank AL, Labotka RJ, Rao S, et al. Haemophilus influenzae Type b Immunization of Children with Sickle Cell Diseases. Pediatrics 1988;82:571–5.
- Feldman S, Gigliotti F, Shenep JL, Roberson PK, Lott L. Risk of Haemophilus influenzae type b disease in children with cancer and response of immunocompromised leukemic children to a conjugate vaccine. J Infect Dis 1990;161:926–31.
- American Academy of Pediatrics. Immunizations for Native American children. Pediatrics 1999;104:564–7.
- Galil K, Singleton R, Levine O, et al. Reemergence of invasive Haemophilus influenzae type b disease in a well-vaccinated population in remote Alaska. J Infect Dis 1999;179:101–6.
- Johnson PDR, Hanlon M, Isaacs D, Gilbert GL. Differing antibody responses to Haemophilus influenzae type b after meningitis or epiglottitis. Epidemiol Infect 1996;116:21–6.
- Ladhani S, Heath P, Ramsay ME, et al. Long-term immunological follow-up of children with Haemophilus influenzae serotype b vaccine failure in the United Kingdom. Clin Infect Dis 2009;49:372–80.
- Breukels MA, Spanjaard L, Sanders LAM, Rijkers GT. Immunological characterization of conjugated Haemophilus influenzae type b vaccine failure in infants. Clin Infect Dis 2001;32:1700–5.
- American Academy of Pediatrics. Immunization in special clinical circumstances. In: Pickering L, Baker C, Kimberlin D, Long S, eds. Red Book: 2012 report of the Committee on Infectious Diseases. Elk Grove Village, IL; 2012:69–71.
- Feldman S. Interchangeability of vaccines. Pediatr Infect Dis J 2001;20:S23–9.
- Scheifele D, Law B, Mitchell L, Ochnio J. Study of booster doses of two Haemophilus influenzae type b conjugate vaccines including their interchangeability. Vaccine 1996;14:1399–406.
- Reid R, Santosham M, Croll J, Thompson C, Newcomer W, Siber GR. Antibody response of Navajo children primed with PRP-OMP vaccine to booster doses of PRP-OMP vs. HbOC vaccine. Pediatr Infect Dis J 1993;12:812–5.
- CDC. Recommended childhood immunization schedule—United States, 1998. MMWR 1998;47:8–12.
- GlaxoSmithKline. MENHIBRIX (meningococcal groups C and Y and Haemophilus b tetanus toxoid conjugate vaccine) [Package insert]. Available at http://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM308577.pdf.
- Shapiro ED, Wald ER. Efficacy of rifampin in eliminating pharyngeal carriage of Haemophilus influenzae type b. Pediatrics 1980;66:5–8.
- McCracken G, Ginsburg CM, Zweighaft TC, Clahsen J. Pharmacokinetics of rifampin in infants and children: relevance to prophylaxis against Haemophilus influenzae type b disease. Pediatrics 1980;66:17–21.
- Glode M, Daum R, Boies E, Ballard T, Murray M, Granoff D. Effect of rifampin chemoprophylaxis on carriage eradication and new acquisition of Haemophilus influenzae type b in contacts. Pediatrics 1985;76:537–42.
- Band J, Faser D, Ajello G. Prevention of Haemophilus influenzae type b disease. JAMA 1984;251:2381–6.
- Bruce MG, Zulz T, DeByle AP, et al. Haemophilus influenzae serotype a invasive disease, Alaska, USA, 1983–2011. Emerg Infect Dis 2013;19:932–7.
- Hammitt LL, Block S, Hennessy TW, et al. Outbreak of invasive Haemophilus influenzae serotype a disease. Pediatr Infect Dis J 2005;24:453–6.
* A list of the members of the Work Group appears on page 14.
† Infants with persistent complement component deficiencies, those with functional or anatomic asplenia (including sickle cell disease), healthy infants in communities with a meningococcal disease outbreak for whom vaccination is recommended, and infants traveling to or residing in areas with hyperendemic or epidemic meningococcal disease.
§ Infants who do not require ongoing management for serious infection, metabolic disease, or acute renal, cardiovascular, neurologic, or respiratory tract illness and who demonstrate a clinical course of sustained recovery and pattern of steady growth (90).
FIGURE 1. Estimated annual incidence* of invasive Haemophilus influenzae type b (Hib) disease in children aged <5 years — United States, 1980–2012
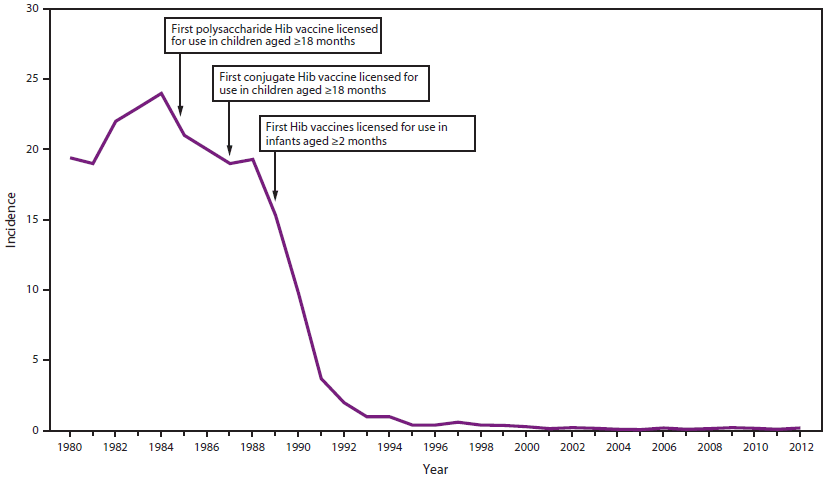
Sources: 1980–1997: National Bacterial Meningitis Reporting System and National Notifiable Diseases Surveillance (NNDSS) data; Adams WG, Deaver KA, Cochi SL, et al. Decline of childhood Haemophilus influenzae Type b (Hib) disease in the Hib vaccine era. JAMA 1993;269:221–6; CDC. Progress toward elimination of Haemophilus influenzae type b disease among infants and children—United States, 1987–1995. MMWR 1996;45:901–6; CDC. Progress toward elimination of Haemophilus influenzae type b disease among infants and children—United States, 1987–1997. MMWR 1998;47:993–8. 1998–2009: NNDSS and Active Bacterial Core Surveillance (ABCs) data. 2010–2012: ABCs cases estimated to the U.S. population.
* Per 100,000 population.
Alternate Text: The figure shows the estimated incidence per 100,000 population of invasive Haemophilus influenzae Type b (Hib) disease in children aged <5 years in the United States during 1980-2012. Incidence declined precipitously following licensure of vaccines for use in children aged ≥18 months and infants aged ≥2 months.
FIGURE 2. Estimated annual incidence* of invasive Haemophilus influenzae Type b infection in children aged <5 years — United States, 2000–2012
Sources: National Notifiable Diseases Surveillance and Active Bacterial Core Surveillance (ABCs) data.
* Per 100,000 population.
Alternate Text: The figure shows the estimated incidence per 100,000 population of invasive Haemophilus influenzae Type b disease in children aged <5 years in the United States during 2000-2012. Incidence fell below the Healthy People 2020 goal of 0.27 cases per 100,000 population for all years shown since 2000 (it was slightly over the goal in 2000).
FIGURE 3. Percentage of children aged <5 years with cases of invasive Haemophilus influenzae type b (Hib) disease,* by vaccine status — United States 2002–2012
Sources: Active Bacterial Core surveillance system and National Notifiable Diseases Surveillance System.
* N = 265. An additional 57 children aged <5 years with Hib had unknown vaccine status and were excluded.
† Among those with age-appropriate vaccine status, 41% were too young to complete the primary series, 16% completed the primary series, and 43% completed the full series.
Alternate Text: The figure shows the percentage of children aged <5 years with cases of invasive Haemophilus influenzae type b (Hib) disease in the United States during 2002-2012, by vaccine status. Among those with age-appropriate vaccine status, 41% were too young to complete the primary series, 16% completed the primary series, and 43% completed the full series.
|
TABLE 1. Haemophilus influenzae type b (Hib) conjugate vaccines licensed and available in the United States as of January 2014 |
|||||
|---|---|---|---|---|---|
|
Vaccine product |
Manufacturer |
Trade Name |
Components |
Primary series |
Booster dose |
|
Monovalent vaccine |
|||||
|
PRP-OMP*,† |
Merck & Co, Inc |
PedvaxHIB |
PRP conjugated to OMP |
2, 4 mos |
12–15 mos |
|
PRP-T |
sanofi pasteur |
ActHIB |
PRP conjugated to tetanus toxoid |
2, 4, 6 mos |
12–15 mos |
|
PRP-T |
GlaxoSmithKline |
Hiberix |
PRP conjugated to tetanus toxoid |
Not licensed for primary series |
12–15 mos§ |
|
Combination vaccine |
|||||
|
PRP-OMP-HepB*,† |
Merck & Co, Inc |
Comvax |
PRP-OMP + hepatitis B vaccine |
2, 4 mos |
12–15 mos |
|
DTaP-IPV/PRP-T |
sanofi pasteur |
Pentacel |
DTaP-IPV + PRP-T |
2, 4, 6 mos |
15–18 mos¶ |
|
MenCY/PRP-T** |
GlaxoSmithKline |
MenHibRix |
MenCY + PRP-T |
2, 4, 6 mos |
12–15 mos |
|
Source: Adapted from American Academy of Pediatrics. Haemophilus influenzae infections. Pickering L, Baker C, Kimberlin D, Long S, eds. Red book: 2012 report of the Committee on Infectious Diseases. Elk Grove Village, IL: American Academy of Pediatrics; 2012:345–52. * If a PRP-OMP vaccine is not administered as both doses in the primary series, or if there is uncertainty about which products were administered previously, a third dose of Hib conjugate vaccine is needed to complete the primary series. † Preferred vaccine for American Indian/Alaska Native children. § To facilitate timely booster vaccination, Hiberix can be administered as early as age 12 months, in accordance with Hib vaccination schedules for routine and catch-up immunization (CDC. Licensure of a Haemophilus influenzae type b [Hib] vaccine [Hiberix] and updated recommendations for use of Hib vaccine. MMWR 2009;58:1008–9) ¶ The booster dose may be administered as early as age 12 months, provided that at least 6 months have elapsed since the third dose. ** Recommendations for the MenCY component of MenCY/PRP-T have been published previously (CDC. Infant meningococcal vaccination: Advisory Committee on Immunization Practices (ACIP) recommendations and rationale. MMWR 2013;62:52–4). |
|||||
|
TABLE 2. Guidance for Haemophilus influenzae type b (Hib) vaccination in high-risk groups |
|
|---|---|
|
High-risk group* |
Hib vaccine guidance |
|
Patients aged <12 mos |
Follow routine Hib vaccination recommendations |
|
Patients aged 12–59 mos |
If unimmunized or received 0 or 1 dose before age 12 mos: 2 doses, 8 wks apart |
|
Patients aged <60 months undergoing chemotherapy or radiation therapy† |
If routine Hib doses administered ≥14 days before starting therapy: revaccination not required |
|
Patients aged ≥15 mos undergoing elective splenectomy |
If unimmunized:§ 1 dose prior to procedure¶ |
|
Asplenic patients aged >59 mos and adults |
If unimmunized:§ 1 dose |
|
HIV-infected children aged ≥60 mos |
If unimmunized:§ 1 dose |
|
HIV-infected adults |
Hib vaccination is not recommended |
|
Recipients of hematopoietic stem cell transplant, all ages |
Regardless of Hib vaccination history: 3 doses (at least 4 wks apart) beginning 6–12 mos after transplant |
|
* Persons with functional or anatomic asplenia, HIV infection, immunoglobulin deficiency including immunoglobulin G2 subclass deficiency, or early component complement deficiency, recipients of a hematopoietic stem cell transplant, and those receiving chemotherapy or radiation therapy for malignant neoplasms. † Some experts suggest conducting serologic testing for these patients (Source: Rubin LG, Levin MJ, Ljungman P, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis 2013;[Epub ahead of print] doi: 10.1093/cid/cit684). § Patients who have not received a primary series and booster dose or at least 1 dose of Hib vaccine after 14 months are considered unimmunized. ¶ Some experts suggest vaccination at least 14 days before the procedure (Sources: CDC. General recommendations on immunization: recommendations of the Advisory Committee on Immunization Practices [ACIP]. MMWR 2011;60[No. RR-2]; CDC. Recommendations of the Advisory Committee on Immunization Practices (ACIP): use of vaccines and immune globulins in persons with altered immunocompetence. MMWR 1993;42[No. RR-4]; Rubin LG, Levin MJ, Ljungman P, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis 2013;[Epub ahead of print] doi: 10.1093/cid/cit684.) Some experts suggest administering a dose prior to elective splenectomy regardless of prior vaccination history (Source: American Academy of Pediatrics. Haemophilus influenzae infections. In: Pickering L, Baker C, Kimberlin D, Long S, eds. Red book: 2012 report of the Committee on Infectious Diseases. Elk Grove Village, IL: American Academy of Pediatrics; 2012:345–52). |
|
Advisory Committee on Immunization Practices
Membership as of February 21, 2013
Chair: Jonathan Temte, MD, PhD, University of Wisconsin School of Medicine and Public Health, Madison, Wisconsin
Executive Secretary: Larry Pickering, MD, National Center for Immunization and Respiratory Diseases, CDC, Atlanta, Georgia.
Members: Nancy Bennett, MD, University of Rochester School of Medicine and Dentistry, Rochester, New York; Joseph Bocchini Jr, MD, Louisiana State University Health Sciences Center, Shreveport, Louisiana; Douglas Campos-Outcalt, MD, University of Arizona College of Medicine, Phoenix, Arizona; Tamera Coyne-Beasley, MD, University of North Carolina, Chapel Hill, North Carolina; Jeffrey Duchin, MD, University of Washington, Seattle, Washington; Kathleen Harriman, PhD, California Department of Public Health, Richmond, California; Lee Harrison, MD, University of Pittsburgh, Pittsburgh, Pennsylvania; Renée Jenkins, MD, Howard University School of Medicine, District of Columbia; Ruth Karron, MD, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland; Wendy Keitel, MD, Baylor College of Medicine, Houston, Texas; Sara Rosenbaum, JD, Georgetown University, District of Columbia; Lorry Rubin, MD, Steven and Alexandra Cohen Children's Medical Center of New York, New Hyde Park, New York; Mark Sawyer, MD, University of California at San Diego, California; Marietta Vázquez, MD, Yale University School of Medicine, New Haven, Connecticut.
Ex Officio Members: Vito Caserta, MD, Health Resources and Services Administration, Rockville, Maryland; Jesse Geibe, MD, Department of Defense, CDC, Atlanta, Georgia; Bruce Gellin, MD, National Vaccine Program Office, District of Columba; Richard Gorman, MD, National Institutes of Health, Bethesda, Maryland; Amy Groom, MPH, Indian Health Service, Albuquerque, New Mexico; Mary Beth Hance, Centers for Medicare and Medicaid Services, Baltimore, Maryland; Linda Kinsinger, MD, Department of Veterans Affairs, Durham, North Carolina; Wellington Sun, MD, Food and Drug Administration, Bethesda, Maryland.
Liaison Representatives: American Academy of Family Physicians, Jamie Loehr, MD, Ithaca, New York; American Academy of Pediatrics, Michael Brady, MD, Ohio State University, Columbus, Ohio, David Kimberlin, MD, Birmingham, Alabama; American Academy of Physician Assistants, Marie-Michèle Léger, MPH, Alexandria, Virginia; American College Health Association, James C. Turner, MD, Charlottesville, Virginia; American College of Obstetricians and Gynecologists, Laura Riley, MD, Boston, Massachusetts; American College of Physicians, Gregory Poland, MD, Rochester, Minnesota; American Geriatrics Society, Kenneth Schmader, MD, Durham, North Carolina; America's Health Insurance Plans, Mark Netoskie, MD, Houston, Texas; American Medical Association, Litjen Tan, PhD, Chicago, Illinois; American Nurses Association, Katie Brewer, MSN, Silver Springs, Maryland; American Osteopathic Association, Stanley Grogg, DO, Tulsa, Oklahoma; American Pharmacists Association, Stephan L. Foster, PharmD, Memphis, Tennessee; Association of Immunization Managers, Kelly Moore, MD, Nashville, Tennessee; Association for Prevention Teaching and Research, W. Paul McKinney, MD, Louisville, Kentucky; Association of State and Territorial Health Officials, José Montero, MD, Concord, New Hampshire; Biotechnology Industry Organization, Clement Lewin, PhD, Cambridge, Massachusetts; Canadian National Advisory Committee on Immunization, Bryna Warshawsky, MDCM, London, Ontario, Canada; Council of State and Territorial Epidemiologists, Christine Hahn, MD, Boise, Idaho; Department of Health, United Kingdom, David M. Salisbury, MD, London, United Kingdom; Healthcare Infection Control Practices Advisory Committee, Alexis Elward, MD, St Louis, Missouri; Infectious Diseases Society of America, Kathleen Neuzil, MD, Seattle, Washington; National Association of County and City Health Officials, Matthew Zahn, MD, Santa Ana, California; National Association of Pediatric Nurse Practitioners, Patricia Stinchfield, MPH, St Paul, Minnesota; National Foundation for Infectious Diseases, William Schaffner, MD, Nashville, Tennessee; National Medical Association, Patricia Whitley-Williams, MD, New Brunswick, New Jersey; National Vaccine Advisory Committee, Walter Orenstein, MD, Atlanta, Georgia; Pharmaceutical Research and Manufacturers of America, Damian A. Braga, Swiftwater, Pennsylvania; Society for Adolescent Health and Medicine, Amy Middleman, MD, Houston, Texas; Society for Healthcare Epidemiology of America, Harry Keyserling, MD, Atlanta, Georgia.
The ACIP Meningococcal and Haemophilus influenzae b Work Group
Membership as of February 21, 2013
Chair: Lorry Rubin, MD, Steven and Alexandra Cohen Children's Medical Center of New York, New Hyde Park, New York
Members: William Atkinson, MD; Carol Baker, MD, Baylor College of Medicine, Houston, Texas; Michael Brady, MD, Ohio State University, Columbus, Ohio; Douglas Campos-Outcalt, MD, University of Arizona College of Medicine, Phoenix, Arizona; Richard Clover, MD, University of Louisville School of Public Health, Louisville, Kentucky; Kristen Ehresmann, MPH, Minnesota Department of Health, St. Paul, Minnesota; Rachel Herlihy, MD, Association of Immunization Managers, Denver, Colorado; Lucia Lee, MD, Food and Drug Administration, Rockville, Maryland; Martin Luta, MD, Delaware Division of Public Health, Dover, Delaware; Michael Marcy, MD, University of Southern California School of Medicine, Los Angeles, California; W. Paul McKinney, MD, Association for Prevention Teaching and Research, Louisville, Kentucky; Cody Meissner, MD, Tufts University School of Medicine, Boston, Massachusetts; Amy Middleman, MD, Society for Adolescent Health and Medicine, Houston, Texas; Karen O'Brien, US Army Training and Doctrine Command, Fort Monroe, Virginia; Paul Offit, MD, Children's Hospital of Philadelphia, Philadelphia, Pennsylvania; Georges Peter, MD, Rhode Island Hospital, Providence, Rhode Island; David M. Salisbury, MD, Department of Health, London, United Kingdom; William Schaffner, MD, National Foundation for Infectious Diseases, Nashville, Tennessee; David Stephens, MD, Emory University School of Medicine, Atlanta, Georgia; James C. Turner, MD, American College Health Association, Charlottesville, Virginia; Marietta Vázquez, MD, Yale University School of Medicine, New Haven, Connecticut.
Contributors (CDC): Yabo Akinsanya-Beysolow, MD, Elizabeth C. Briere, MD, Thomas Clark, MD, Amanda C. Cohn, MD, Jonathan Duffy, MD, Jessica MacNeil, MPH, Nancy Messonnier, MD, Ismael Ortega-Sanchez, PhD, Shannon Stokley, MPH.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All MMWR HTML versions of articles are electronic conversions from typeset documents.
This conversion might result in character translation or format errors in the HTML version.
Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr)
and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S.
Government Printing Office (GPO), Washington, DC 20402-9371;
telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to
mmwrq@cdc.gov.


