FIGURE 1. Number of wild poliovirus (WPV) cases, by type, month of onset, and type of supplementary immunization activity --- India, January 2006--September 2011
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail.
Progress Toward Poliomyelitis Eradication --- India, January 2010--September 2011
The Global Polio Eradication Initiative was launched in 1988 (1). In 1995, when eradication activities were initiated in India, an estimated 50,000 polio cases were occurring each year (2). By 2006, transmission of indigenous wild poliovirus (WPV) had been interrupted in all countries except India, Afghanistan, Pakistan, and Nigeria (1). During 2006--2009, India annually reported 559 to 874 cases of confirmed WPV, with cases centered in the northern states of Uttar Pradesh and Bihar (3). These cases accounted for 43% of confirmed cases of WPV reported worldwide during this period. However, in 2010, only 42 WPV cases were reported in India, and in 2011, only one WPV case had been confirmed as of October 31. This report updates previous reports (2,3) and summarizes progress toward polio eradication in India during January 2010--September 2011. Throughout India, the most recent confirmed WPV type 3 (WPV3) case occurred on October 22, 2010, in Jharkhand, and the most recent confirmed WPV type 1 (WPV1) case occurred on January 13, 2011, in West Bengal; WPV2 has not been reported in India since 1999. Importation of WPV into India is a risk, and undetected low-level WPV transmission is a possibility, requiring high vaccination coverage in all states, continued focus on children in migrant and underserved populations, sensitive surveillance for prompt detection of any WPV, and preparedness to mount a robust emergency vaccination campaign in response to any WPV cases.
Immunization Activities
The most recent population-based survey from 2009 found that nationwide routine vaccination coverage with 3 doses of trivalent oral polio vaccine (tOPV) was 70% among children aged 12--23 months (4). This percentage is similar to the reported estimates of nationwide routine vaccination coverage in 2010 with 3 doses of tOPV by age 12 months (5). The survey estimates for coverage in Bihar (62%; increased from 51% in 2006) and Uttar Pradesh (54%; increased from 50% in 2006) were among the lowest in the country, whereas coverage was higher in Jharkhand (70%) and West Bengal (74%) (4).
For the past several years, increased attention in India during supplementary immunization activities (SIAs)* has focused on vaccinating 1) populations at high risk for polio in areas where polio has been endemic and 2) large populations of migrants, who contributed to the persistence and spread of WPV transmission (3). In January 2010, bivalent OPV (bOPV) types 1 and 3 was introduced for use in SIAs, largely replacing monovalent OPVs (mOPVs) (3).
SIAs conducted in India during 2010--2011 included two national immunization days each year. In addition, six subnational immunization days (SNIDs) and four large-scale (multidistrict) mop-up† activities were conducted during 2010, and six SNIDs and one large-scale mop-up were conducted during January--September 2011 (Figure 1). The response to the WPV case in West Bengal in 2011 included three SIAs, using a combination of mOPV type 1 and bOPV conducted within 7 weeks of notification of the case (the first SIA was held 7 days after detection), followed by monthly SIAs using bOPV for 6 months (Figure 2).
Monitoring data§ are used to estimate the proportion of children missed in each SIA. In 2011, the mean percentage of missed children in the general population aged <2 years based on these SIA surveys was 0.3% in Bihar, 1.8% in Uttar Pradesh, 3.7% in Jharkhand, and 6.1% in West Bengal. Efforts to identify and vaccinate urban slum dwellers and specific migrant populations (e.g., construction laborers, nomads, and brick kiln workers) were enhanced further during 2010--2011. The percentage of missed children per SIA in the migrant population aged <2 years, based on surveys conducted after SIA rounds in 2011 in nine states with large numbers of these populations, was 0.4%--12.3% (mean: 2.3%). In Uttar Pradesh, the mean percentage of children missed in migrant populations (1.3%) was comparable to the mean percentage of children missed in the general population aged <2 years (1.8%). In West Bengal, the mean percentage of children missed in migrant populations aged <2 years (7.3%) was higher than the overall mean among migrant populations in eight other states (2.0%) and remains higher than the mean percentage of children missed in the general population in that state (6.1%).
WPV Surveillance
Acute flaccid paralysis (AFP) surveillance. In India, the national nonpolio AFP (NPAFP) rate,¶ a proxy measure of polio surveillance system sensitivity, was 12.7 per 100,000 children aged <15 years in 2010 and 12.1 per 100,000 (annualized) during January--September 2011. During that period, the highest state-level NPAFP rates were in Bihar (37.9) and Uttar Pradesh (23.9); three of 35 states and union territories had NPAFP rates <2 per 100,000. Adequate stool specimen collection** in India was 83% in 2010 and 84.1% during January--September 2011; in five states, adequate specimen collection was <80% during January--September 2011.††
Environmental surveillance. Weekly testing of wastewater for poliovirus began in Mumbai in June 2001 and in Delhi in May 2010, and biweekly testing began in Patna, Bihar, in April 2011. Both WPV1 and WPV3 were detected in wastewater at Delhi sites during 2010; WPV3 was last detected in July 2010, and WPV1 was last detected in August 2010. The most recent WPV from wastewater in India was WPV1 isolated in November 2010 in Mumbai. No WPV has been isolated from Patna wastewater. All WPV1 and WPV3 isolates from wastewater during 2010--2011 were genetically related to WPV1 and WPV3 circulating in central Bihar during 2009.
WPV Epidemiology
During 2010, a total of 42 WPV cases (18 WPV1 and 24 WPV3) were reported in India in 17 districts in seven states (Figure 3). During January--September 2011, only one WPV case (WPV1) was reported in India, in West Bengal, compared with 40 WPV cases (17 WPV1 and 23 WPV3) from 17 districts in seven states during the same period during 2010. All WPV1 isolates from patients during 2010--2011 were related genetically to WPV circulating in central Bihar during 2009.
The most recent confirmed WPV1 case in India occurred on January 13, 2011, in a child from a community in which the rate of vaccine refusal was high during previous SIAs in Howrah District, West Bengal. The most recent confirmed WPV3 case occurred on October 22, 2010, in Jharkhand. During 2010, simultaneous transmission of WPV1 and WPV3 occurred in Jharkhand and West Bengal around their common border (Figures 2 and 3). However, the WPV1 isolated from the patient in Howrah was not directly related genetically to WPV1 circulating at the border in Jharkhand and West Bengal during 2010, but was related genetically to WPV circulating in Bihar in September 2009 and also isolated from wastewater in Delhi in August 2010.
Among the 43 WPV cases reported during 2010--2011, 30 (70%) occurred among children aged <2 years. Of these children, six (14%) had received 1--3 OPV doses, 11 (26%) had received 4--7 doses, 24 (56%) had received >7 doses, one (2%) had unknown vaccination status, and one (2%) had not received any OPV doses (the child with WPV1 in West Bengal in January 2011).
Reported by
Ministry of Health and Family Welfare, Government of India. National Polio Surveillance Project, World Health Organization, India; Regional Poliovirus Laboratory Network, Immunization and Vaccine Development Dept, World Health Organization Regional Office for South-East Asia. Polio Eradication Department, World Health Organization, Geneva, Switzerland. United Nations Children's Fund (UNICEF), New York, New York. Div of Viral Diseases, National Center for Immunization and Respiratory Diseases and Global Immunization Div, Center for Global Health, CDC. Corresponding contributor: Jalaa' Abdelwahab, joa4@cdc.gov, 212-326-7672.
Editorial Note
During 2010 and 2011, India made substantial progress toward polio eradication. A year has passed since the last confirmed WPV3 case, and >9 months have passed since the last confirmed WPV1 case. The absence of any reported WPV cases since January, including during much of the June-November high-transmission season, is unprecedented. WPV was last detected in sewage in Delhi in August 2010 and in Mumbai in November 2010. The subsequent lack of detection of WPV in any samples from any site is further indication that WPV transmission might have been interrupted. No WPV cases have been reported for >17 months and >12 months in the previously polio-endemic states of Uttar Pradesh and Bihar, respectively. If no WPV is identified throughout the high-transmission season in 2012, India will be regarded as polio-free. This would put the World Health Organization South-East Asia Region, of which India is a member, on track to be certified polio-free as early as 2014.
The introduction of bOPV in SIAs beginning in January 2010 likely contributed substantially to the simultaneous reduction in WPV1 and WPV3 cases in India. Previous SIAs were conducted predominantly using mOPV type 1 and occasionally using mOPV type 3; a clinical trial demonstrated the superiority of bOPV compared with tOPV and noninferiority compared with mOPV types 1 and 3 (6). The special attention focused on vaccination coverage of children in high-risk endemic areas and migrant populations during the last several years likely increased and sustained the levels of immunity needed to stop WPV transmission. Rapid response SIAs contributed to successfully stopping WPV transmission after report of the case in Howrah, West Bengal. This response, led by the government and partners,§§ included massive mobilization of human and financial resources, revision of detailed subdistrict SIA operational plans, retraining of vaccinators and supervisors, increased community outreach and mobilization, and enhanced monitoring in the outbreak areas. Of note is that routine vaccination coverage improved in both Bihar and Uttar Pradesh over the last several years at the same time the two states conducted almost monthly SIAs and frequent large-scale mop-ups.
AFP surveillance indicators have reached or greatly exceeded targets in the majority of states and territories since 2005. Continued vigilance is needed to ensure that all states and union territories reach targets for surveillance indicators and that high-risk populations are adequately included to achieve the highest sensitivity required for detecting any WPV circulation. Appropriately targeted environmental surveillance can be more sensitive in detecting low-level WPV circulation than AFP surveillance (7). Sewage sampling is ongoing in Mumbai, Delhi, and Patna, Bihar, and is planned to begin in Kolkata, West Bengal, late in 2011.
Despite the absence of WPV cases in India since January 2011, the risk remains for WPV circulation among migrant populations and residents of high-risk areas in western Uttar Pradesh and central Bihar and in migrant populations in other states. In West Bengal, families within certain migrant populations continue to have higher proportions of undervaccinated children than families in migrant populations and the general population aged <2 years in other states, according to 2011 directed surveys of these populations.
Although India has served as a reservoir for importation to neighboring countries and some distant countries (8), the country also is at risk for WPV importations from other polio-affected areas. The recent polio outbreak in neighboring China resulting from WPV importation from Pakistan (9) is a reminder of the need for continued vigilance to ensure high population immunity in all states (with specific focus on migrant populations) along with rapid response SIAs after any detected WPV cases. Elimination of WPV in India will establish that WPV transmission can be interrupted even in the most challenging of settings, remove the threat of importation from India, and provide impetus to the Global Polio Eradication Initiative goal of interrupting all WPV transmission globally by the end of 2012.
References
- CDC. Progress toward interruption of wild poliovirus transmission---worldwide, January 2010--March 2011. MMWR 2011;60:582--6.
- CDC. Progress toward poliomyelitis eradication---India, 1998. MMWR 1998;47:778--81.
- CDC. Progress toward poliomyelitis eradication---India, January 2009--October 2010. MMWR 2010;59:1581--5.
- United Nations Children's Fund. 2009 coverage evaluation survey. New Delhi, India: United Nations Children's Fund; 2010. Available at http://www.unicef.org/india/health_6679.htm. Accessed October 31, 2011.
- World Health Organization--United Nations Children's Fund routine immunization coverage estimates. Available at http://apps.who.int/immunization_monitoring/en/globalsummary/timeseries/tswucoveragepol3.htm. Accessed October 28, 2011.
- Sutter RW, John TJ, Jain H, et al. Immunogenicity of bivalent types 1 and 3 oral poliovirus vaccine: a randomised, double-blind, controlled trial. Lancet 2010;376:1624--5.
- Deshpande JM, Shetty SJ, Siddiqui ZA. Environmental surveillance system to track wild poliovirus transmission. Appl Environ Microbiol 2003;69:2919--27.
- CDC. Outbreaks following wild poliovirus importations---Europe, Africa, and Asia, January 2009--September 2010. MMWR 2010;59:1393--9.
- World Health Organization. Outbreak news: confirmed international spread of wild poliovirus from Pakistan. Wkly Epidemiol Rec 2011;86:437--8.
* SIAs are mass campaigns conducted over a period of days in which 1 dose of OPV is administered to all children aged <5 years, regardless of vaccination history. Surveillance data analysis determines the geographic extent of campaigns (i.e., national or subnational).
† Mop-up rounds are intensive house-to-house SIAs conducted in a limited area (groups of districts) with evidence of recent transmission.
§ SIA monitoring data are obtained from systematic surveys conducted after every SIA in high-risk areas to identify children aged <2 years who were missed with vaccination.
¶ The NPAFP rate is the number of AFP cases not associated with WPV per 100,000 children aged <15 years. India's operational target for each district is two or more AFP cases per 100,000.
** The percentage of reported AFP cases with two stool specimens collected within 14 days of paralysis onset with at least 24 hours between the two specimens (target: ≥80%).
†† The eight polio laboratories in India processed 109,057 stool specimens during 2010 and 85,161 stool specimens during January--September 2011.
§§ World Health Organization, United Nations Children's Fund (UNICEF), Rotary International and CORE Group.
What is already known on this topic?
India is one of four countries (the others are Afghanistan, Nigeria, and Pakistan) where wild poliovirus (WPV) remains endemic. Until 2010, most polio cases in India were reported in Uttar Pradesh and Bihar, two states with low routine vaccination coverage, lower vaccine effectiveness than elsewhere, and large migrant populations that require frequent supplementary immunization activities (SIAs) to control WPV transmission.
What is added by this report?
As of October 31, only one confirmed WPV case had been reported in India during January--September 2011, compared with 40 WPV cases during the same 9-month period in 2010. A year has passed since the most recent WPV3 case in India and more than 9 months since the last case of WPV1. This unprecedented finding is corroborated by the lack of any WPV isolation from wastewater samples since November 2010, and likely resulted from a combination of factors, including introduction of bivalent oral poliovirus vaccine types 1 and 3 in SIAs in 2010, targeting of migrant populations, and rapid outbreak response.
What are the implications for public health practice?
In India, the risk for importation or undetected WPV transmission remains, particularly in migrant populations. To ensure interruption of all WPV transmission, strong surveillance and high population immunity are needed in all states (with specific focus on migrant populations), as well as rapid response SIAs after any detected WPV cases. Elimination of WPV in India will establish that WPV transmission can be interrupted even in the most challenging of settings, remove the threat of importation from India, and provide impetus to the Global Polio Eradication Initiative goal of interrupting all WPV transmission.
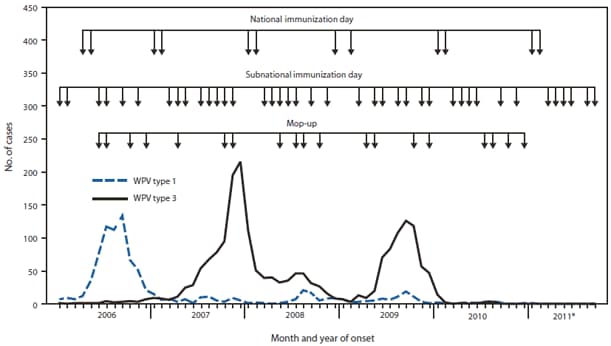
* Data as of October 31, 2011.
Alternate Text: The figure above shows the number of wild poliovirus (WPV) cases, by type, month of onset, and type of supplementary immunization activity in India during January 2006-September 2011. Supplementary immunization activities (SIA) conducted in India during 2010-2011 included two national immunization days each year. In addition, six sub¬national immunization days (SNIDs) and four large-scale (multidistrict) mop-up activities were conducted during 2010, and six SNIDs and one large-scale mop-up were conducted during January-September 2011.
FIGURE 2. Number of wild poliovirus (WPV) cases (n = 17), by type, week of onset, and vaccine used in supplementary immunization activities --- selected districts, India, January 2010---September 2011
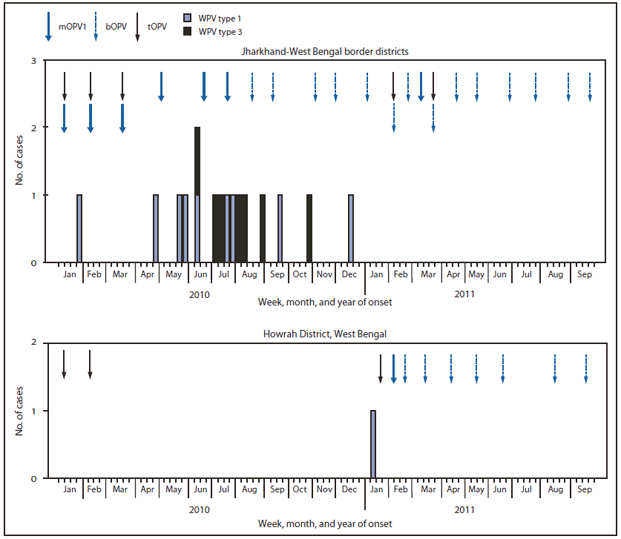
Abbreviations: mOPV1 = monovalent oral poliovirus vaccine type 1; bOPV = bivalent oral poliovirus vaccine; tOPV = trivalent oral poliovirus vaccine.
Alternate Text: The figure above shows the number of wild poliovirus (WPV) cases (n = 17), by type, week of onset, and vaccine used in supplementary immunization activities in selected districts in India during January 2010-September 2011. The response to the WPV case in West Bengal in 2011 included three SIAs, using a combination of monovalent oral poliovirus vaccine (mOPV) type 1 and bivalent oral poliovirus vaccine (bOPV) conducted within 7 weeks of notification of the case (the first SIA was held 7 days after detection), followed by monthly SIAs using bOPV for 6 months.
FIGURE 3. Wild poliovirus (WPV) cases (N = 43), by type --- India and selected states, 2010 and 2011*
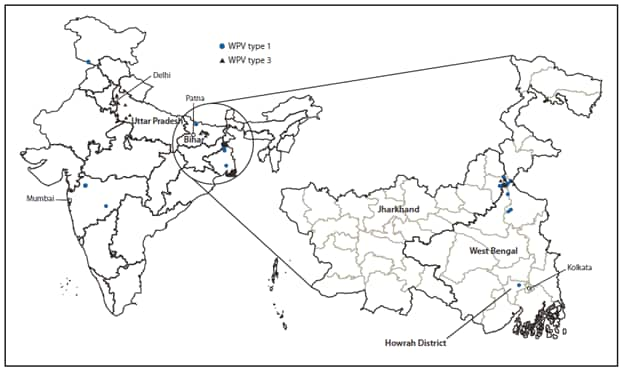
* Data as of October 31, 2011.
Alternate Text: The figure above shows wild poliovirus (WPV) cases (N = 43), by type in India and selected states during 2010 and 2011. During 2010, a total of 42 WPV cases (18 WPV1 and 24 WPV3) were reported in India in 17 districts in seven states.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All MMWR HTML versions of articles are electronic conversions from typeset documents.
This conversion might result in character translation or format errors in the HTML version.
Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr)
and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S.
Government Printing Office (GPO), Washington, DC 20402-9371;
telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to
mmwrq@cdc.gov.


