Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail.
Progress Toward Poliomyelitis Eradication --- India, January 2009--October 2010
India is one of only four countries (including Afghanistan, Nigeria, and Pakistan) where wild poliovirus (WPV) transmission has never been interrupted (1). Historically, WPV transmission in India has centered largely in Uttar Pradesh and Bihar, two states with low routine vaccination coverage, large migrant and remote populations, and lower relative vaccine effectiveness than other areas of the country (2--4). However, during a 9-month period from November 2009 to August 2010, no WPV type 1 (WPV1) cases were reported in Uttar Pradesh or Bihar. This report summarizes the substantial progress made in India toward polio eradication during January 2009--October 2010, according to data reported as of December 4, and updates previous reports (2,4). During January--October 2010, only 40 WPV cases were confirmed in India, a 94% decrease from the 626 WPV cases confirmed during the same period in 2009; the decrease likely resulted, in large part, from the introduction of bivalent oral poliovirus vaccine types 1 and 3 (bOPV). Increasingly important contributors to WPV transmission are large migrant subpopulations; surveys have indicated that up to 11% of children aged <5 years in these subpopulations were missed during supplementary immunization activities (SIAs). Interruption of all WPV transmission in India will require maintaining high levels of immunity in Uttar Pradesh and Bihar and additional efforts directed toward children in migrant subpopulations that are not vaccinated as readily during SIAs.
Immunization Activities
Using population-based survey data, India estimated nationwide routine coverage with 3 doses of oral poliovirus vaccine (OPV) at 66% among children aged 12--23 months during 2007--2008, the most recent years for which coverage data were available (5). Routine coverage estimates in Bihar (53%) and Uttar Pradesh (40%) were among the lowest in the country (5).
SIAs* conducted in India during 2009--2010 (Figures 1 and 2) included two national immunization days (NIDs) each year. In addition, seven subnational immunization days (SNIDs) and four large-scale (multidistrict) mop-up† activities were conducted during 2009, and five SNIDs and three large-scale mop-ups were conducted during January--October 2010 (Figure 1). After introduction of bOPV in January 2010, six SIAs were conducted using bOPV.
In 2010, SIA monitoring data§ indicated >99% coverage among children aged <2 years in Bihar and >97% in Uttar Pradesh. After enhanced efforts during 2009--2010 to identify specific areas in other states where migrant populations resided, directed surveys conducted with specific migrant subpopulations (e.g., construction laborers, nomads, and brick kiln workers) after SIA rounds indicated that 3%--11% of children aged <5 years had been missed. In Uttar Pradesh during 2010, surveys after SIAs indicated that, on average, 4.1% of children in the migrant subpopulations, compared with 2.2% missed among children aged <5 years in the general population.
WPV Surveillance
Acute flaccid paralysis (AFP) surveillance. The national nonpolio AFP rate,¶ a measure of surveillance system sensitivity, was 11.4 per 100,000 children aged <15 years in 2009 and 11.1 per 100,000 (annualized) during January--October 2010. The highest state-level nonpolio AFP rates were in Bihar (33.9) and Uttar Pradesh (22.8) in 2010. Adequate stool specimen collection** in India was 83% in 2009 and 84% during January--October 2010.
Environmental surveillance. Wastewater testing for poliovirus began in Mumbai in January 2001 and in Delhi in May 2010. Although WPV was isolated frequently from samples taken in Mumbai in previous years, no WPV was detected in Mumbai wastewater in 2010. Environmental testing during May--August 2010 detected both WPV1 and WPV type 3 (WPV3) in wastewater at Delhi sites. Genetic analysis has suggested WPV circulation within Delhi was linked to 2009 WPV1 and WPV3 Bihar isolates. No WPV has been detected in environmental samples since mid-August.
Laboratory network. During January--October 2010, >90% of stool specimens submitted for virus isolation had laboratory results reported within 14 days of specimen receipt. The mean interval from onset of paralysis and confirmation of WPV isolation was 24 days.††
WPV Epidemiology
During all of 2009, a total of 741 WPV cases were reported in India from 56 districts in nine states of 35 states/union territories in India (Figure 3). During January--October 2010, a total of 40 WPV cases had been reported from 17 districts in seven states, a 94% decrease from the 626 WPV cases from 52 districts in nine states during the same reporting period in 2009. Among the 40 WPV cases reported in 2010, 28 (70%) occurred in children aged <2 years. Six (15%) of the 40 children had received 1--3 OPV doses, eight (20%) had received 4--7 doses, and 25 (63%) had received >7 doses; one child had unknown vaccination status. In Uttar Pradesh and Bihar, 19 cases had been reported from 10 districts; all of these patients had received >7 OPV doses. According to data reported as of December 4, 2010, during January--October 2010, a total of 17 WPV1 and 23 WPV3 cases were confirmed, representing a 78% decrease from 76 WPV1 cases and a 96% decrease from 550 WPV3 cases confirmed during the same period in 2009.
WPV1. In 2009, a total of 80 WPV1 cases were reported (including one case with both WPV1 and WPV3 isolated) from 35 districts in six states. During January--October 2010, a total of 17 WPV1 cases were reported from seven districts in five states. WPV1 isolates related to 2009 Bihar WPV1 strains have been isolated from AFP patients with onset of paralysis during January--October 2010 in West Bengal (five patients), Jharkhand (three), and Maharashtra (five). In addition, WPV1 strains circulating in Bihar during 2009 were associated with a WPV1 case in Jammu and Kashmir in 2010 after importation into Punjab in 2009. The most recent WPV1 case in India had onset on September 21 in West Bengal.
The last confirmed WPV1 case in Uttar Pradesh was in a patient with onset of paralysis on November 13, 2009. In Bihar, no WPV1 cases were reported from October 30, 2009, to August 7, 2010. Subsequently, three cases have been reported in a single Bihar district bordering Nepal (Champaran East), with onset in the most recent case on September 1. The recent Bihar outbreak began after an outbreak was identified in Nepal in May 2010 immediately across the border from Champaran East; WPV1 isolates from both areas are related to WPV1 strains circulating in Bihar during 2009.
WPV3. In 2009, a total of 661 WPV3 cases were reported from 47 districts in eight states; 569 (86%) were from Uttar Pradesh, and 79 (12%) were from Bihar. During January--October 2010, a total of 23 cases were reported from 12 districts in five states, compared with 550 cases from 43 districts in seven states during the same 10-month period in 2009. Of the 23 cases reported during January--October 2010, 10 (43%) were from Uttar Pradesh, six (26%) from Bihar, four (17%) from Jharkhand, two (9%) from West Bengal, and one (4%) from Haryana. The most recent WPV3 case in India had onset on August 31 in Jharkhand.
Reported by
Ministry of Health and Family Welfare, Government of India. National Polio Surveillance Project, World Health Organization, India; Regional Poliovirus Laboratory Network, Immunization and Vaccine Development Dept, World Health Organization Regional Office for South-East Asia. Div of Viral Diseases and Global Immunization Div, National Center for Immunization and Respiratory Diseases; CV Cardemil, MD, EIS Officer, CDC
Editorial Note
During 2009--2010, India made substantial progress toward polio eradication. The absence of reported WPV1 cases in Uttar Pradesh and Bihar for 9 months during November 2009--August 2010 was unprecedented; Uttar Pradesh has remained free of detected WPV cases since April 2010. For the first 10 months of 2010, the total number of WPV cases in India reached a new low at 40, compared with 626 cases during the same reporting period in 2009.
The introduction of bOPV in SIAs beginning in January 2010 likely contributed substantially to the simultaneous reduction in WPV1 and WPV3 cases. Previous SIAs were conducted predominantly using monovalent oral poliovirus vaccine type 1 (mOPV1) and occasionally monovalent oral poliovirus vaccine type 3 (mOPV3); trivalent oral poliovirus vaccine (tOPV) was used less often because higher type-specific seroconversion per dose has been observed with mOPV formulations than with tOPV (3,6). A recent clinical trial demonstrated the superiority of bOPV compared with tOPV and noninferiority compared with mOPV1 and mOPV3 (7). Once supplies became available, bOPV became the predominant formulation used in SIAs. Preliminary data from August 2010 seroprevalence studies among infants aged 6--7 months in high-risk areas of Uttar Pradesh and Bihar indicate that, after bOPV introduction, seroprevalence against WPV3 increased and high levels of seroprevalence against WPV1 were maintained (Enterovirus Research Center, Mumbai, India, unpublished data, 2010).
Appropriately targeted environmental surveillance can be more sensitive in detecting low-level WPV circulation than AFP surveillance (8). WPV was last detected in sewage in Mumbai in May 2009 and in Delhi in mid-August 2010, where sewage sampling was initiated in May of this year. The recent lack of detection of WPV in any samples is encouraging; however, sewage sampling in India still is restricted to these two major metropolitan areas.
Despite India's gains in 2010, the risk remains for WPV circulation and reintroduction among migrant populations and residents of high-risk areas in western Uttar Pradesh and central Bihar, primarily because of high population density, weak routine immunization, and suboptimal hygiene and sanitation. Families of certain migrant subpopulations (e.g., construction laborers, nomads, and brick kiln workers) that move regularly to and from Uttar Pradesh and Bihar have higher proportions of undervaccinated children than the general population, according to 2010 directed surveys of these subpopulations and supported by reported vaccination rates among nonpolio AFP case patients.
The risk for persistence of low-level, undetected, WPV transmission among Uttar Pradesh or Bihar residents, or among migrant subpopulations, is a concern. All WPV1 isolates from India and Nepal in 2010 are genetically linked to strains detected in central Bihar in 2009, and the 2010 WPV1 outbreak in Tajikistan was linked to WPV1 from Uttar Pradesh in 2009 (9). OPV-vaccinated children with serologic immunity can excrete WPV, which might contribute to transmission despite high OPV coverage in SIAs (6,10). Moreover, transmission has continued in some areas with recent outbreaks (Maharashtra and West Bengal). WPV could spread to other parts of India with relatively low population immunity months after the last observed case in the outbreak area.
The current high season for polio in India has passed with historically low incidence of WPV cases. Successful interruption of all residual WPV transmission in India will require maintaining high levels of immunity in Uttar Pradesh and Bihar through SIAs and programs to strengthen routine vaccination, along with continued mop-ups to control outbreaks in areas where WPV was reintroduced. SIAs planned for 2011 represent an opportunity to interrupt transmission, provided that high coverage during SIAs is maintained, immediate large-scale mop-ups are conducted in response to any new WPV detected, and focus is continued on vigorous vaccination of migrant subpopulations.
References
- CDC. Progress toward interruption of wild poliovirus transmission---worldwide, 2009. MMWR 2010;59:545--50.
- CDC. Progress toward poliomyelitis eradication---India, January 2006--September 2007. MMWR 2007;56:1187--91.
- Grassly NC, Wenger J, Durrani S, et al. Protective efficacy of a monovalent oral type 1 poliovirus vaccine: a case-control study. Lancet 2007;369:1356--62.
- CDC. Progress toward poliomyelitis eradication---India, January 2007--May 2009. MMWR 2009;58:719--23.
- Ministry of Health and Family Welfare, Government of India. District level household and facility survey. Available at http://www.rchiips.org. Accessed December 3, 2010.
- Sutter RK, Kew OM, Cochi SL. Poliovirus vaccine---live. In: Plotkin S, Orenstein W, Offit P, eds. Vaccines. 5th ed. Philadelphia, PA: Saunders; 2008.
- Sutter RW, John TJ, Jain H, et al. Immunogenicity of bivalent types 1 and 3 oral poliovirus vaccine: a randomised, double-blind, controlled trial. Lancet 2010;376:1624--5.
- Deshpande JM, Shetty SJ, Siddiqui ZA. Environmental surveillance system to track wild poliovirus transmission. Appl Environ Microbiol 2003;69:2919--27.
- CDC. Outbreaks following wild poliovirus importations---Europe, Africa, and Asia, January 2009--September 2010. MMWR 2010;59:1393--9.
- Grassly NC, Jafari H, Bahl S, et al. Asymptomatic wild-type poliovirus infection in India among children with previous oral poliovirus vaccination. J Infect Dis 2010;201:1535--43.
* SIAs are mass campaigns conducted over a period of multiple days in which 1 dose of OPV is administered to all children aged <5 years, regardless of vaccination history. Surveillance data analysis determines the geographic extent of campaigns (i.e., national or subnational).
† Mop-up rounds are intensive house-to-house SIAs conducted in a limited area (groups of districts) with evidence of recent transmission.
§ SIA monitoring data are obtained from systematic surveys conducted after every SIA in high-risk areas to identify children aged <5 years who were missed with vaccination.
¶ The nonpolio AFP rate is the number of AFP cases not caused by WPV per 100,000 children aged <15 years. India's operational target for each district is two or more AFP cases per 100,000.
** The percentage of reported AFP cases with two stool specimens collected within 14 days of paralysis onset (target: ≥80%).
†† The eight polio laboratories in India processed 100,102 stool specimens during 2009 and 91,952 stool specimens during January--October 2010.
What is already known on this topic?
India is one of four countries (including Afghanistan, Nigeria, and Pakistan) where wild poliovirus (WPV) remains endemic; most cases in India in the past have been reported in Uttar Pradesh and Bihar, two states with low routine vaccination coverage, lower vaccine effectiveness than elsewhere, and large migrant subpopulations that require frequent supplementary immunization activities (SIAs) to control WPV transmission.
What is added by this report?
As of December 4, during January--October 2010, a total of 40 WPV cases had been confirmed in 2010, a 94% decrease from the 626 WPV cases during the same 10-month period in 2009; this progress likely resulted in large part from the introduction of bivalent oral poliovirus vaccine types 1 and 3 (bOPV) in SIAs.
What are the implications for public health practice?
Despite the progress in India, the risk for persistent WPV transmission remains, particularly in migrant subpopulations and among residents of Uttar Pradesh and Bihar. To interrupt all WPV transmission, India is taking steps to strengthen routine vaccination and SIA coverage, implement immediate, large-scale mop-ups in response to new cases, target migrant subpopulations, and maintain high levels of immunity in Uttar Pradesh and Bihar.
FIGURE 1. Number of wild poliovirus (WPV) cases, by type, month of onset, and type of supplementary immunization activity --- India, January 2005--October 2010
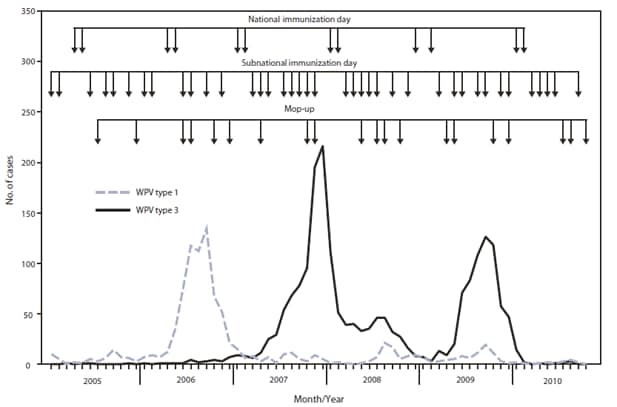
Alternate Text: The figure above shows the number of wild poliovirus (WPV) cases, by type, month of onset, and type of supplementary immunization activity (SIA) in India from January 2005-October 2010. SIAs conducted in India during 2009-2010 included two National Immunization Days (NIDs) each year. In addition, seven Subnational Immunization Days (SNIDs) and four large-scale (multidistrict) mop-up activities were conducted during 2009, and five SNIDs and three large-scale mop ups were conducted during January-October 2010.
FIGURE 2. Number of supplementary immunization activity (SIA)* rounds, by vaccine used and district --- Uttar Pradesh, Bihar, and surrounding areas, India, January 2009--October 2010
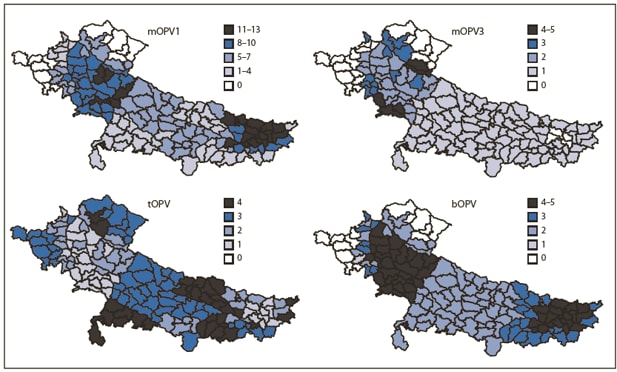
Abbreviations: mOPV1 = monovalent oral poliovirus vaccine type 1; mOPV3 = monovalent oral poliovirus vaccine type 3; tOPV = trivalent oral poliovirus vaccine; bOPV = bivalent oral poliovirus vaccine.
* SIAs are mass campaigns conducted over a period of multiple days in which 1 dose of oral polio vaccine is administered to all children aged <5 years, regardless of vaccination history. Surveillance data analysis determines the geographic extent of campaigns (i.e., national or subnational).
Alternate Text: The figure above shows the number of supplementary immunization activity (SIA) rounds, by vaccine used and district in Uttar Pradesh, Bihar, and surrounding areas in India from January 2009-October 2010. SIAs are mass campaigns conducted over a period of multiple days in which 1 dose of oral polio vaccine is administered to all children aged <5 years, regardless of vaccination history. Surveillance data analysis determines the geographic extent of campaigns (i.e., national or subnational).
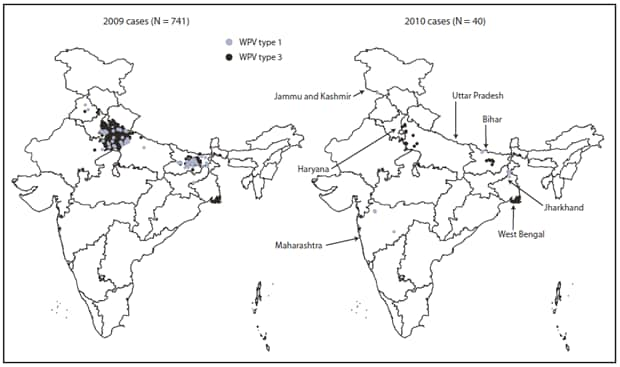
* Data of December 4, 2010.
Alternate Text: The figure above shows Wild poliovirus (WPV) cases, by type in India in 2009 and 2010. During all of 2009, a total of 741 WPV cases were reported in India from 56 districts in nine states.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All MMWR HTML versions of articles are electronic conversions from typeset documents.
This conversion might result in character translation or format errors in the HTML version.
Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr)
and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S.
Government Printing Office (GPO), Washington, DC 20402-9371;
telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to
mmwrq@cdc.gov.


