FIGURE. Number of persons with culture-confirmed group A streptococcus infection (N = 30), by infection type and month of positive culture --- skilled nursing facility, Pennsylvania, 2009--2010
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail.
Invasive Group A Streptococcus in a Skilled Nursing Facility --- Pennsylvania, 2009--2010
In September 2010, the Pennsylvania Department of Health was notified of a cluster of invasive group A Streptococcus (GAS) infections among residents of a skilled nursing facility specializing in neurologic and pulmonary care. The Montgomery County Health Department, the Pennsylvania Department of Health, and CDC conducted an investigation to identify additional cases and GAS carriers, assess risk factors and infection prevention practices, implement control measures, and prevent further infections. The investigators determined that, during October 12, 2009--September 22, 2010, 10 residents at the facility had noninvasive GAS infection, and 13 had invasive GAS infection; two residents with invasive infection died. An additional seven culture-confirmed GAS infections could not be characterized as cases because the residents' symptoms were unknown. Staff members and residents were screened by culture to identify GAS carriers, who were then administered antibiotics. Multiple infection prevention deficiencies were noted at the facility, including ineffective hand hygiene practices among staff members. Education on infection prevention practices was provided, and active surveillance was implemented. Invasive GAS outbreaks in long-term--care facilities often result in high death rates (1). Long-term--care facilities, including skilled nursing facilities, should investigate single cases of invasive GAS infection and ensure that infection prevention measures are fully implemented.
Identification of Cases and Carriers
On September 29, 2010, the Pennsylvania Department of Health was notified of three laboratory-confirmed invasive GAS infections in a skilled nursing facility. Review of Pennsylvania's reportable disease surveillance data, which includes invasive GAS cases, identified eight additional invasive GAS infections at the facility since October 2009. The 150-bed facility specializes in ventilator weaning, spinal cord injury care, and short-term rehabilitation. Cases were defined as symptomatic, culture-confirmed GAS infections in residents of the facility during January 1, 2009--September 30, 2010. Signs or symptoms consistent with GAS infection included fever ≥100.5°F (≥38.1°C), upper-respiratory symptoms (e.g., cough or sore throat), and purulent discharge, skin redness, or swelling. Cases were categorized as invasive (GAS cultured from a normally sterile site) or noninvasive. Reports of positive GAS cultures from facility residents were obtained from three area hospitals and the skilled nursing facility's reference laboratory and were cross-referenced with the facility's census.
Case-finding confirmed 11 cases of invasive GAS disease that previously had been reported to the county and identified two other invasive and 10 noninvasive cases (five respiratory, four wound, and one eye infection) during October 12, 2009--September 22, 2010. No cases were identified for the periods January 1--October 11, 2009, and September 23--30, 2010. Seven additional residents had culture-positive test results from nonsterile sites; however, these results could not be characterized as cases because symptom data were unavailable in the patients' medical records (Figure).
The 23 GAS patients ranged in age from 31 to 97 years (median: 55 years); 52% were male, and 48% were black. All 13 patients with invasive GAS were hospitalized and had GAS isolated from blood cultures obtained on the date of hospitalization; two hospitalized patients also had GAS isolated from respiratory specimens. Two deaths were associated with invasive GAS infection (case-fatality rate: 15%). Among the 13 patients, at the time of hospitalization, six had fever, six had respiratory distress, and seven had tachycardia. Eleven patients had leukocytosis identified, four had pneumonia based on radiography, and one had cellulitis.
Carriers were defined as residents or staff members who had GAS cultured from nonsterile sites with no clinical evidence of GAS infection at the time of culturing. Beginning October 5, 2011, a total of 436 persons (139 residents and 297 staff members) were screened for GAS carriage. Specimens were obtained from the oropharynx, wounds, and the skin surrounding patients' tracheostomies, gastrostomies, jejunostomies, central lines, and indwelling urinary catheters. One (0.7%) resident had a positive GAS culture from his urinary catheter insertion site, and four (1.3%) staff members (two nurses and two housekeepers) had positive GAS oropharyngeal cultures. All five GAS carriers received antibiotics recommended for GAS decolonization (2); staff members identified as GAS carriers were furloughed until they had received 48 hours of antibiotic therapy. Facility admissions were suspended during October 5--18 until carriers had been treated and infection prevention audits completed.
Assessment of Infection Prevention Practices
Beginning October 13, 2010, audits of infection prevention practices were performed in all clinical units of the facility. Interviews were conducted with administrative personnel, the facility's full-time infection preventionist, and staff members; front-line staff members were observed in daily practice. An assessment of available infection prevention resources (e.g., gloves, sinks, and alcohol-based hand rub dispensers) was completed. Compliance with contact precautions and hand hygiene was measured by using a mobile telephone application.* Hand hygiene was defined as hand washing or alcohol-based hand rub use, in accordance with CDC guidelines (3) and the World Health Organization's five opportunities for hand hygiene (4).
Multiple infection prevention deficiencies were observed. Hand hygiene compliance was 32% (17 of 53 observed opportunities), and the facility lacked recommended hand-hygiene resources. Although alcohol-based hand rub dispensers were located in every resident's room, the manufacturer had stopped producing refills approximately 1 month before the evaluation, and staff members reported that even when refills had been available, in-room dispensers were often empty or malfunctioning. Sinks were located in resident rooms but not in central locations (e.g., nursing stations). During wound care treatments, potentially infectious materials (e.g., open, unused gauze and biohazard bags containing discarded wound supplies) were moved from room to room. Contact precaution signage was not implemented in a timely manner after identification of infectious organisms.
At the time of the investigation, a full-time infection preventionist, who had been hired in August 2010, was in the process of assessing and improving infection prevention practices. The infection preventionist, along with CDC and state and county health department personnel, provided training to staff members regarding standardized infection prevention practices, including hand hygiene, timely contact precaution implementation, and proper wound care practices. After training and placement of functioning alcohol-based hand rub dispensers on a wall in every resident room, hand hygiene compliance improved to 70% (56 of 80 observed opportunities).
Typing to Determine Strain Relatedness
Eight GAS isolates, three collected from patients during September 2010 and all five isolates collected from carriers during October, were available for emm typing at CDC to assess strain relatedness.† Four of the isolates (from one patient, one resident carrier, and the two nurse carriers) were emm 89.0; two isolates from patients were emm 11.0, and the isolates from the two housekeeper carriers were emm 3.6 and emm 2.0.
Associations with Risk Factors
In October 2010, a matched case-control study was performed at the facility to identify associations between potential risk factors and GAS infection. All 23 patients with invasive and noninvasive disease were included as case-patients. Residents were chosen randomly from the resident census as control subjects if they had no evidence of positive GAS culture or symptoms consistent with GAS infection in the 3 days before or after the matched case-patient's positive culture date. Control subjects were matched 3:1 to case-patients. Chart reviews were conducted for case-patients and control subjects by using a standardized data collection form focusing on potential risk factors during the month before the date of positive GAS culture.
Bivariate analysis indicated that GAS case-patients were significantly more likely than control subjects to have the following risk factors: length of stay ≤10 months (matched odds ratio [mOR] = 7.0), having a wound (mOR = 4.6), having two or more wounds (mOR = 7.0), having an indwelling urinary catheter (mOR = 3.9), residing in a pulmonary unit (mOR = 3.7), receiving physical therapy (mOR = 4.1), and receiving occupational therapy (mOR = 2.3) (Table). Statistically significant variables from bivariate analysis were considered for inclusion in a multivariate model to assess their independent association with GAS infection. In the final model, adjusted for physical therapy and length of stay, case-patients were significantly more likely to have two or more wounds (adjusted odds ratio = 3.9; 95% confidence interval = 1.1--13.3) than control subjects.
Subsequent Developments
In December 2010, two new invasive GAS infections were identified in residents of the pulmonary units, prompting rescreening of all 59 pulmonary unit residents and 112 staff members who had direct contact with the newly infected residents. Three residents (5.1%) and four staff members (3.6%) were newly identified as asymptomatic carriers and administered antibiotics. Facility administrators were advised to engage a consultant to assist the infection preventionist in optimizing implementation of previously recommended measures. Since December 2010, no additional cases have been identified.
Reported by
Julie Marsden, MA, Montgomery County Health Dept; Lisa Dettinger, George Fraser, Bur of Laboratories; Kirsten Waller, MD, Maria Moll, MD, Andre Weltman, MD, Atmaram Nambiar, MD, Stephen Ostroff, MD, Aimee Palumbo, MPH, Pennsylvania Dept of Health. Bernard Beall, PhD, Streptococcus Laboratory, Chris Van Beneden, MD, Div of Bacterial Disease, National Center for Immunization and Respiratory Diseases; Carolyn Gould, MD, Nimalie Stone, MD, Div of Healthcare Quality Promotion, National Center for Emerging and Zoonotic Infectious Diseases; Neil Gupta, MD, Allison Longenberger, PhD, EIS officers, CDC. Corresponding contributor: Allison Longenberger, c-alongenb@pa.gov, 717-787-3350.
Editorial Note
GAS infections are transmitted by person-to-person contact or by respiratory droplets and can result in severe invasive infection. Identification of 13 cases of invasive GAS infection during a 12-month period represents one of the largest and most prolonged invasive GAS outbreaks in a nursing facility. The four different emm types from case-patients and carriers and the long duration of the outbreak suggest it did not arise from a single source. The investigation identified infection prevention lapses and an association between two or more wounds and GAS infection.
Consistent with previous GAS outbreaks in nursing facilities, this extended outbreak likely was initiated by separate introductions of GAS into a vulnerable population and perpetuated by suboptimal infection prevention practices (5,6). GAS can colonize in the throats of asymptomatic children and adults; staff members, visitors, or newly admitted residents who are asymptomatic carriers or who have pharyngitis can introduce GAS into long-term--care facilities.
Because of the multiple opportunities for GAS to be introduced into long-term--care facilities and the potential for invasive GAS to cause severe morbidity and mortality among vulnerable populations, facility managers should emphasize the importance of infection prevention and control practices among staff members, particularly hand hygiene and wound care practices, as a crucial means of preventing GAS outbreaks among skilled nursing facility residents (1,7). The findings from this investigation are consistent with previous findings that nonintact skin remains a key risk factor for GAS transmission. However, previously documented risk factors such as advanced age, black race, and having a roommate with GAS infection or carriage (8,9) were not significantly associated with GAS infection in this investigation.
Early identification of invasive GAS infection through surveillance and communication with acute care facilities is essential in preventing and controlling GAS outbreaks and reducing the need for extensive public health responses. Before this facility hired a full-time infection preventionist in August 2010, infection control expertise was limited. Furthermore, the observed infection prevention lapses late in the course of the outbreak demonstrate that the risk for invasive GAS infections was not fully appreciated at this facility, despite ongoing communication from the county health department. This emphasizes the need for improved education regarding the increased risk for severe GAS infections among long-term--care facility residents.
Given the magnitude of this outbreak, the carriage rate among staff members and residents was lower than expected. Although previously published outbreaks in nursing facilities identified carriage rates ranging from 0% to 34% among residents and from 2% to 9% among staff members (1), the low carriage rate in this study might have resulted from deficiencies in swabbing technique, resulting from an effort by staff members to complete a large number of swabbings over a short period, or from other unidentified factors.
This prolonged outbreak occurred in a specialized facility with ventilator-dependent residents and residents with neurologic impairments. The observed infection prevention deficiencies highlight the need for a strong infection prevention program that might include a full-time infection preventionist in facilities providing similarly complex care to vulnerable populations. Skilled nursing facilities should have programs to ensure education, compliance, and resources for infection prevention, with emphasis on hand hygiene, standard and transmission-based precautions, and wound care practices to prevent health-care--associated outbreaks of GAS. Long-term--care facilities, including skilled nursing facilities, should investigate single cases of invasive GAS because of the possibility of unrecognized GAS transmission among staff members and residents. Such investigations should include communication with local and state health departments and acute-care facilities, identification of additional cases through active surveillance and retrospective chart review, identification of potential carriers by screening close contacts of patients and symptomatic health-care workers, and enhanced infection control.
Acknowledgments
Joseph DiMino, DO, Michael Baysinger, MPH, Alexandra Cordova, MS, Michel Masters, MPH, Rachael Quinn, Montgomery County Health Dept; Aaron Smee, MPH, Susan Hammond, MPH, Leah Lind, MPH, Elizabeth Hunt, MPH, Kimberly Warren, MPH, Judi Sedivy, MPH, Christina Russell, James Tait, Kerry Pollard, Jared Seiders, Pennsylvania Dept of Health. Sheryl Lyss, MD, Delois Jackson, MS, Zhongya Li, Lesley McGee, PhD, Christopher Palma, CDC.
References
- Jordan HT, Richards CL, Burton DC, Thigpen MC, Van Beneden CA. Group A streptococcal disease in long-term care facilities: descriptive epidemiology and potential control measures. Clin Infect Dis 2007;45:742--52.
- Prevention of Invasive Group A Streptococcal Infections Workshop participants. Prevention of invasive group A streptococcal disease among household contacts of case patients and among postpartum and postsurgical patients: recommendations from the Centers for Disease Control and Prevention. Clin Infect Dis 2002;35:950--9.
- CDC. Guideline for hand hygiene in health-care settings: recommendations of the Healthcare Infection Control Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. MMWR 2002;51(No. RR-16).
- World Health Organization. WHO guidelines on hand hygiene in health care, 2009. Geneva, Switzerland: World Health Organization; 2009. Available at http://whqlibdoc.who.int/publications/2009/9789241597906_eng.pdf. Accessed October 21, 2011.
- CDC. Nursing home outbreaks of invasive group A streptococcal infections---lllinois, Kansas, North Carolina, and Texas. MMWR 1990;39:577--9.
- Greene CM, Van Beneden CA, Javadi M, et al. Cluster of deaths from group A Streptococcus in a long-term care facility---Georgia, 2001. Am J Infect Control 2005;33:108--13.
- Arnold KE, Schweitzer, JL, Wallace B, et al. Tightly clustered outbreak of group A streptococcal disease at a long-term care facility. Infect Control Hosp Epidemiol 2006;27:1377--84.
- Zurawski CA, Bardsley M, Beall B, et al. Invasive group A streptococcal disease in metropolitan Atlanta: a population-based assessment. Clin Infect Dis 1998;27:150--7.
- Deutscher M, Schillie S, Gould C, et al. Investigation of a group A streptococcal outbreak among residents of a long-term acute care hospital. Clin Infect Dis 2011;52:988--94.
* Additional information available at https://compepi.cs.uiowa.edu/iscrub/home.
† Additional information available at http://www.cdc.gov/ncidod/biotech/strep/m-proteingene_typing.htm.
What is already known on this topic?
Group A Streptococcus (GAS) is transmitted by direct person-to-person contact or by respiratory droplets and can result in severe invasive infections.
What is added by this report?
This large, prolonged outbreak of GAS infection at a skilled nursing facility, with 23 cases, including 13 invasive infections and two deaths, over a 12-month period demonstrated that deficiencies in infection prevention and control can contribute to extended outbreaks of both invasive and noninvasive GAS infection, especially among residents with two or more wounds.
What are the implications for public health practice?
Nursing facilities need strong infection prevention programs, with emphasis on hand hygiene and wound care, to prevent health-care--associated outbreaks of GAS infection. Single cases of invasive GAS should be investigated by long-term--care facilities, with ongoing communication with local and state health departments and acute care facilities. Efforts to prevent and control GAS infections should include methods to identify deficiencies in infection prevention and rapid remediation of any deficiencies.
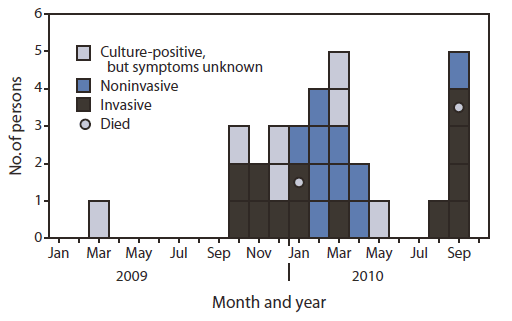
Alternate Text: The figure above shows the number of persons with culture-confirmed group A streptococcus (N = 30), by infection type and month of positive culture at a skilled nursing facility in Pennsylvania during 2009-2010. Thirteen cases were invasive, and 10 were noninvasive; seven culture-positive test results from nonsterile sites could not be characterized as cases because symptom data were unavailable in the patients' medical records.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All MMWR HTML versions of articles are electronic conversions from typeset documents.
This conversion might result in character translation or format errors in the HTML version.
Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr)
and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S.
Government Printing Office (GPO), Washington, DC 20402-9371;
telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to
mmwrq@cdc.gov.


