Procedure for Collection of Patient Swabs for Candida auris
Purpose
C. auris is an emerging multidrug-resistant fungal pathogen that can cause invasive infections with high mortality and has been transmitted in healthcare settings. Identifying persons colonized with C. auris is a key step in containing the spread of C. auris. This document outlines the procedure for collection of swabs from patients to assess for C. auris colonization.
The skin (specifically axilla and groin) appears to be the highest-yield site to swab to identify patients colonized with C. auris. C. auris has also been isolated from swabs taken from the nares, oropharynx, external ear canal, vagina, and rectum. CDC continues to explore whether swabbing of other body sites (e.g., nares, hands) would improve identification of colonized patients.
Safety Considerations
C. auris can survive for weeks on plastic surfaces, has reduced susceptibility to disinfectants with only quaternary ammonia, and can colonize skin of healthy individuals. Therefore, strict BSL2 laboratory safety precautions must be followed when working with this organism. Specifically, cultures should be processed within a BSL2 biosafety cabinet, gloves and lab coats are required, and strong hand hygiene should be enforced. The use of disinfectants with an EPA approval for C. auris are recommended for decontaminations after working with C. auris cultures. For more information about safely working with C. auris, visit the safety considerations page.
Equipment and materials needed
Culture collection and transport system
Patient swabs are taken using a culture collection and transport system consisting of a swab and tube with transport media (pictured below). For example, rayon tip swabs (Fisherfinest Amies Charcoal bacteriology culture collection and transport system; Fisher healthcare, Ontario, Canada) or nylon-flocked swab (BD ESwab collection and transport system; Becton Dickinson and Company, Sparks, MD)”
Disclaimer
Mention of a specific commercial product does not qualify as an endorsement of the product by CDC.
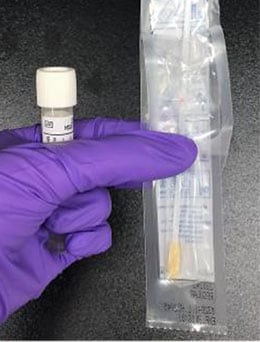
Before beginning, follow institution guidelines for PPE and infection control.
Procedure for collecting the swab
- Open the swab package by grasping the plastic at the opposite end from the soft tip. Leave the swab tip enclosed in the package to prevent contamination.
- Carefully remove the tube from its packaging.
- Pull the swab from its package, being careful not to touch the soft tip. Firmly rub the soft end of the collection swab as described in the next step. Swab both the axilla and groin with the same swab as described below.
- Rub all sides of the swab tip over the left axilla skin surface and then the right, targeting the crease in the skin where the arm meets the body (i.e., swab both armpits, swiping back and forth 5 times per armpit).
- With the same swab used on the axilla, rub both sides of the swab tip over the left groin skin surface, targeting the inguinal crease in the skin where the leg meets the pelvic region and repeat with the right side (i.e., swab the skin of both hip creases, swiping back and forth 5 times per hip crease).
- Remove the cap from the swab collection tube; then place the soft end of the collection swab into the tube. Be careful to keep the cap from touching any materials that may contaminate your sample.
- Snap off the end of the swab at the marked line by bending the plastic handle against the edge of the transport media container.
- Screw on the tube cap. You may need to adjust it until the snapped end of the swab slides into place in the center of the cap.
- Write specimen information on the tube label or apply patient identification label.
- Send or ship immediately to a testing laboratory.
- Specimens should be tested within 4 days of collection, or as otherwise indicated by testing laboratory.
Disclaimer
This test has not been cleared or approved by the FDA. The performance characteristics have been established by CDC Mycotic Diseases Laboratory.