ACIP Evidence to Recommendations Framework for use of PRIORIX for Prevention of Measles, Mumps, and Rubella
Question: Should MMR vaccine (PRIORIX, GSK) be recommended as an option according to currently recommended schedules and off-label uses to prevent measles, mumps, and rubella?
Population: Persons ≥6 months of age
Intervention: GSK PRIORIX
Comparison: Existing MMR vaccine licensed in the US (M-M-R II, Merck)
Outcomes:
Prevention of measles, mumps, and rubella
Short-term humoral immunity
Persistence of humoral immune response
Reactogenicity grade ≥3
Serious adverse events
Additional adverse events of interest (febrile seizures, aseptic meningitis, immune thrombocytopenic purpura)
Background:
Measles, mumps, and rubella are vaccine-preventable acute viral diseases with a large burden of disease worldwide. Due to achieving and maintaining high measles, mumps, and rubella (MMR) vaccination coverage, endemic measles and rubella/congenital rubella syndrome were eliminated in the United States in 2000 and 2004 respectively. Even with high vaccination coverage, there are pockets of un- and under-vaccinated individuals at risk for import-associated cases and outbreaks of measles. These outbreaks continue to occur, with 22 outbreaks and 1,249 cases reported in 2019, the most US cases reported in a single year since 1992 (1).
Mumps, while not eliminated, experienced a >99% reduction in cases in the United States due to the high, sustained vaccination coverage. However, since 2006, the United States experienced an increase in mumps outbreaks in different close-contact settings with high intensity exposure, including schools, universities, church groups, workplaces, and others. The largest of these outbreaks resulted in nearly 3,000 cases in 2016-2017 (2). High MMR vaccination coverage helps limit the size, duration, and spread of mumps outbreaks (2).
Previously, only one MMR combination vaccine was licensed and available in the United States: M-M-R II, Merck & Co., Inc. The Advisory Committee on Immunization Practices (ACIP) recommends routine vaccination of children with two doses of MMR, with the first dose at age 12-15 months and the second at age 4-6 years. Additional recommendations include catch-up vaccination of persons without acceptable evidence of immunity; for infants aged 6-11 months during measles outbreaks; before international travel for infants aged 6-11 months and travelers aged ≥12 months who have not received two doses of MMR; for measles post-exposure prophylaxis; and a third dose during mumps outbreaks for persons identified by public health authorities as being part of a group or population at increased risk for acquiring mumps because of an outbreak (3, 4).
PRIORIX (GSK) is a MMR combination vaccine that was approved in the United States in June 2022 for use in persons aged 12 months and older. Considering availability of the newly licensed MMR vaccine, the ACIP MMR Vaccine Work Group assessed data related to potential benefits and harms of including PRIORIX as an option for MMR vaccination, according to the existing recommended schedules and off label uses*. No changes to the existing ACIP recommendations for the use of MMR vaccine were proposed.
Problem
| Criteria | Work Group Judgments | Evidence | Additional Information |
|---|---|---|---|
| Is the problem of public health importance?
Is prevention of measles, mumps, and rubella a problem of public health importance?
|
Yes | ACIP has recognized measles, mumps, and rubella as problems of public health importance and currently recommends routine and catch-up vaccination to prevent these diseases.
Given this information and the existing ACIP recommendations for vaccination for prevention of measles, mumps, and rubella, the Work Group is of the opinion that the problem is of public health importance.
|
Attaining and maintaining high 2-dose MMR coverage (≥90% since 1996) has led to endemic measles, rubella, and congenital rubella syndrome elimination in the US, and low levels of mumps.
Despite high 2-dose vaccine coverage, measles and mumps continue to cause locally acquired and importation-related cases and outbreaks.
|
Benefits and Harms
| Criteria | Work Group Judgments | Research Evidence [15] | Additional Information |
|---|---|---|---|
| How substantial are the desirable anticipated effects?
For prevention of measles, mumps and rubella (seroresponse), how substantially different are the desirable anticipated effects of PRIORIX compared with M-M-R II?
|
Minimal | No randomized controlled trial (RCT) efficacy data were available directly comparing PRIORIX and M-M-R II for the prevention of measles, mumps, and rubella. However, a retrospective case-control study from the United Kingdom during 2006-2018 showed similar vaccine effectiveness and disease prevention with PRIORIX, as compared to other MMR vaccines (5).
Of the 16 RCTs identified in the systematic literature review with data on immunogenicity and safety, 13 reported immunogenicity data – four at the licensed US potency of PRIORIX (6-9), and nine at a lower PRIORIX potency used in other countries (10-18). Of the four studies at the US potency, seroprotective antibody levels were achieved for all three antigens in all studies with no significant difference in anti-measles, anti-mumps, or anti-rubella geometric mean concentrations after the first or second dose among PRIORIX versus M-M-R II recipients.
The evidence suggests that seroresponse of PRIORIX is non-inferior compared with seroresponse of M-M-R II.
|
In all 13 RCTs with immunogenicity data available, antibodies were ≥8.8 fold higher than the correlate of protection for measles (200 mIU/mL), ≥4.2 fold higher than the rubella correlate (10 IU/mL). For mumps, where a correlate of protection has not been established, the antibody level was ≥3.3 fold higher than the mumps seroconversion threshold (10 UI/mL). |
| How substantial are the undesirable anticipated effects?
For the outcomes of serious and mild adverse events, how substantially different are the undesirable anticipated effects of PRIORIX compared with M-M-R II?
|
Minimal | Serious adverse events (SAEs)§ related to administration of PRIORIX were assessed using four RCTs trials at the licensed US potency of PRIORIX and one Cochrane review with PRIORIX at any potency (6-9). Four additional studies and one additional systematic review addressed additional adverse events of interest (rate of febrile seizures, aseptic meningitis, and immune thrombocytopenic purpura [ITP]) (19-24). The RCTs and the other safety studies included compared the safety outcome of PRIORIX administration with that of M-M-R II administration.
In the 4 RCTs, safety profiles among 1,960 subjects receiving one or two doses of PRIORIX at US licensure potency were compared with those among 933 subjects receiving one or two doses of M-M-R II. The subjects were aged 12 months to 12 years with 90% aged 12 to 15 months. The frequency of vaccine-related SAEs was 0.0%-0.2% of subjects receiving PRIORIX, compared with 0.0%-0.3% receiving M-M-R II (6-9). No significant difference in frequency of vaccine-related SAEs was observed within each individual study; pooled estimates were not calculated.
Reactogenicity grade 3** was compared in 2,113 subjects receiving one or two doses of PRIORIX and 980 subjects receiving one or two doses of M-M-R II. The frequency of complaints grade 3 reactogenicity was 0.3%-10.3% for those receiving PRIORIX compared with 0.5%-12.3% for those receiving M-M-R II (7-9). No significant difference in frequency of vaccine-related SAEs was observed within each individual study; pooled estimates were not calculated.
The rate of febrile seizures is highest in the 6 to 11 days following vaccination for all MMR vaccines and is estimated to be 3.3-8.7 per 10,000 doses based on two studies conducted in the United Kingdom which included both PRIORIX and M-M-R II (20, 21). In the clinical trials conducted in the United States by GSK with PRIORIX of any potency after receipt of a first dose of MMR (PRIORIX or M-M-R II) at age 12-15 months, the rate of febrile seizures among 8,386 PRIORIX recipients was 9.5 per 10,000 (95% confidence interval [CI] 4.4, 19.6) compared to 14.0 (95% CI 5.2, 34.8) among 3,561 M-M-R II recipients. These studies included age-appropriate vaccine co-administration and all found the differences in rates of febrile seizures to be non-significant between the two vaccines (8, 9, 11, 13). Similarly, the time course of fever was comparable for both vaccines across all studies, with most instances observed 5-12 days post vaccination (GSK, personal communication, 2022).
The association between MMR vaccination and aseptic meningitis has been demonstrated in vaccines containing the Urabe or Leningrad-Zagreb mumps strains (25). No evidence of association was found for vaccines containing Jeryl Lynn or Jeryl-Lynn derived mumps strains, which is the mumps strain included in both M-M-R II and PRIORIX (19, 22, 25).
ITP is associated with the receipt of live attenuated measles vaccines (19, 21, 23-25). In the 4 clinical trials conducted with PRIORIX at the US potency, one case of ITP was identified among 1,960 PRIORIX recipients and one case among 933 M-M-R II recipients. From a prior postmarketing study conducted in the United States, the rate of ITP after M-M-R II is estimated at 2.5 per 100,000 doses (21). However, strain or vaccine formulation specific data is sparse. Based on the clinical trials and the literature (19, 21, 23-25), the rates of ITP after vaccination were considered similar for PRIORIX and M-M-R II.
PRIORIX may result in little to no difference in serious adverse events or other adverse events of interest examined when compared with M-M-R II.
|
|
| Do the desirable effects outweigh the undesirable effects?
Does the balance between desirable effects and undesirable effects favor PRIORIX or M-M-R II?
|
Favors both | The desirable effects of PRIORIX, including additional options for providers and patients, and security of the supply chain, outweigh the undesirable effects as there is no evidence of a difference in safety or immunogenicity for PRIORIX as compared to M-M-R II. |
Acceptability
| Criteria | Work Group Judgments | Research Evidence | Additional Information |
|---|---|---|---|
| Is the intervention acceptable to key stakeholders?
Is PRIORIX acceptable to key stakeholders?
|
Yes | A survey of 400 pediatricians and 400 family medicine practitioners was conducted during May 24-June 6, 2022. Participants were asked a number of questions including on their current MMR vaccination practices and barriers to having a second MMR product. The key findings from the survey as related to acceptability were:
These results highlighted the importance of addressing interchangeability and off-label usage of vaccines for PRIORIX acceptance. To assess acceptability (and feasibility) a focus group with state health department immunization managers was conducted in May 2022 to identify potential barriers to PRIORIX use from this stakeholder’s perspective. Participants noted that the majority of Vaccine for Children and vaccine program orders are based on provider’s demand and that this is the main factor driving new vaccine adoption.
With ACIP recommending fully interchangeability and the same off-label usage of the two vaccine products, there were no major barriers to acceptability for PRIORIX.
|
Equity
| Criteria | Work Group Judgments | Research Evidence | Additional Information |
|---|---|---|---|
| What would be the impact on health equity?
What would be the impact of PRIORIX compared to M-M-R II on health equity?
|
Probably no impact | M-M-R II and PRIORIX will have the same Vaccine for Children (VFC) price. Given this and the similarities in formulation and storage (26, 27), the work group considered that there would probably be no impact on health equity for PRIORIX compared to M-M-R II. |
Feasibility
| Criteria | Work Group Judgments | Research Evidence | Additional Information |
|---|---|---|---|
| Is the intervention feasible to implement?
Is PRIORIX feasible to implement?
|
Yes | Between 2000 and mid-2003, the United States experienced occasional shortages of a number of routinely recommended vaccines including MMR. These shortages were due to two voluntary interruptions to manufacturing operations by Merck, both of which took longer to resolve than anticipated and were significant enough that the ACIP recommendations had to be temporarily modified, including suspension of the second dose of MMR in cases of insufficient vaccine quantities (28).
Redundancy in supply is a critical component of sustainable public health.
In the focus group with state health department immunization managers indicated above, adding PRIORIX into the routine immunization system, vaccine access, distribution, storage and handling, and implementation of communication and education strategies were not considered barriers. Participants welcomed the idea of having another brand available in case of outbreaks or manufacturing interruptions. In addition, one participant noted the benefit of having a gelatin free vaccine (PRIORIX) for religious populations.
Given this evidence, the work group considered that an additional MMR vaccine (PRIORIX) that is safe and non-inferior to the existing MMR (M-M-R II) vaccine could be beneficial in maintaining measles and rubella elimination and mitigating mumps outbreaks in the United States as well as assuring supplier diversity.
|
An important point to note is that MMRV (combination MMR and varicella vaccine), accounts for roughly 13% (ranging from 5% to 24%) of first doses and roughly 80% (52% to 98%) of second doses in this childhood vaccine series. (CDC, Immunization Services Division, unpublished data, 2022) |
Based on similarities in vaccine components, schedule and vaccine mechanism of action, the ACIP MMR Work Group perceived that the domains of Values and Resource Use for PRIORIX are comparable with Values and Resource Use of M-M-R II.
Balance of Consequences
Desirable consequences clearly outweigh undesirable consequences in most settings.
Recommendation
MMR vaccine (PRIORIX, GSK) should be recommended as an option according to the existing MMR recommended schedules and off-label uses to prevent measles, mumps, and rubella.
Additional considerations
Given the similarities in potency and vaccine components, the evidence from the clinical trials, and literature review, the Work Group considers PRIORIX and M-M-R II fully interchangeable, including all off-label uses. PRIORIX may be administered in any situation in which a measles, mumps, and rubella-containing vaccine is indicated.
*Off-label uses include: Both M-M-R II and PRIORIX: children aged 6-11 months who will travel or live abroad or during measles outbreaks and third dose of MMR in persons previously vaccinated with 2 doses of a mumps virus–containing vaccine who are identified by public health authorities as being part of a group or population at increased risk for acquiring mumps because of an outbreak. Additionally, measles post-exposure prophylaxis for PRIORIX.
†Methods for assessing potential benefits and harms: Data on the outcomes of interest were summarized based on findings from a systematic review of the literature in PubMed, Medline (Ovid), Embase, Scopus, Cochrane databases, and clinicaltrials.gov published from January 1, 1980 through May 31, 2022. Search terms included (“Measles-Mumps-Rubella Vaccine”and (“Priorix” or “MMR vaccine” or (“GlaxoSmithKline*” or “GSK”) and “MMR*”) or “GSK-MMR” or “MMR-RIT” or “SB-MMR”) and “Safe*” or “effective*” or “efficacy” or “immun*” or “interchangeab*” or “inter-changeab*” or “adverse” or “M-M-R II” or “Merck” or “evidence*” or (“review” or “meta*”).
To be included in the review, a study had to present immunogenicity or disease endpoints or safety data on PRIORIX. Studies were included if they met all of the following criteria: 1) Randomized controls trials or systematic reviews and meta-analyses, 2) vaccine administered to persons ≥6 months of age, 3) at least 1 dose of MMR (PRIORIX) vaccine as the intervention, 4) at least 1 dose of MMR (M-M-R II) vaccine as the comparator, and 5) were not animal studies. SAEs and reactogenicity grade ≥3 were evaluated only in studies conducted at or above the licensed US potency of PRIORIX. Additional adverse events and immunogenicity were evaluated at any potency of PRIORIX.
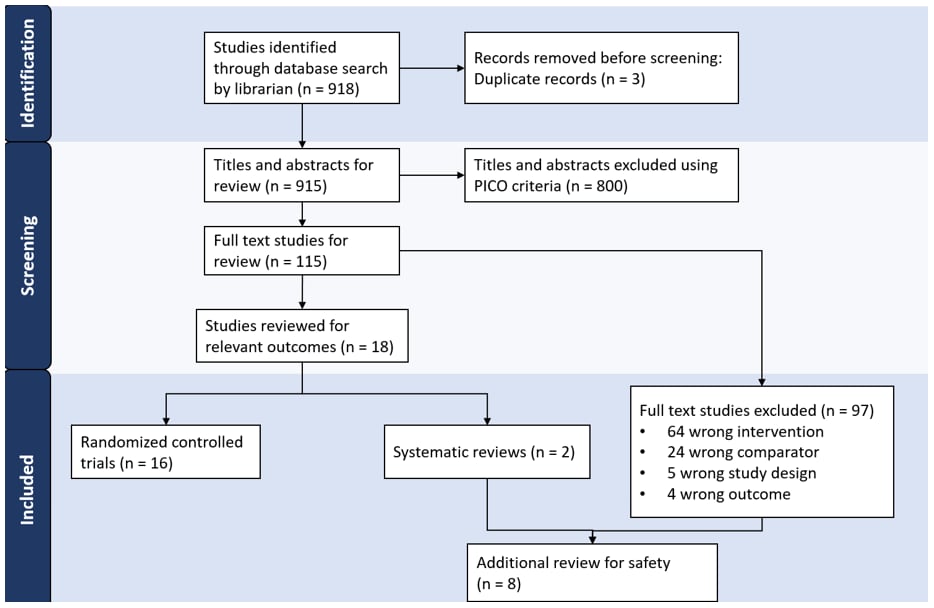
The search identified 918 studies of which 115 underwent full text review. Of those, 16 RCTs, two systematic reviews, and six studies with a different study design or comparator vaccine were included (FIGURE 1).
FIGURE 1: PRISMA Flow Diagram: Identification of PRIORIX Studies
§Serious adverse event is defined as an undesirable experience associated with the vaccine that results in death, hospitalization, disability or requires medical or surgical intervention to prevent a serious outcome.
**Grade 3 intensity was defined as crying when the limb was moved or the limb was spontaneously painful (pain), event preventing normal activity (drowsiness), crying inconsolably or preventing normal activity (irritability), not eating at all (loss of appetite). Note that these are percentage of total complaints, not individuals; one individual participant could report multiple complaints.
References
- Mathis AD, Clemmons NS, Redd SB, Pham H, Leung J, Wharton AK, et al. Maintenance of measles elimination status in the United States for 20 years despite increasing challenges. Clin Infect Dis. 2021 Nov 26.
- Marlow M, Leung J, Marin M, Hickman C, Clemmons N, Shepersky L, et al. Chapter 9: Mumps. Centers for Disease Control and Prevention; 2021.
- Marin M, Marlow M, Moore KL, Patel M. Recommendation of the Advisory Committee on Immunization Practices for Use of a Third Dose of Mumps Virus–Containing Vaccine in Persons at Increased Risk for Mumps During an Outbreak. Morbidity Mortal Wkly Rep. 2018;67(1):33-8.
- McLean HQ, Fiebelkorn AP, Temte JL, Wallace GS, Prevention CfDCa. Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: summary recommendations of the Advisory Committee on Immunization Practices (ACIP). Mmwr Recomm Reports Morbidity Mortal Wkly Rep Recomm Reports. 2013;62(RR-04):1-34.
- Povey M, Aris E, Cheuvart B, Hall G, Cohet C, Willame C. Effectiveness of “Priorix” Against Measles and Mumps Diseases in Children Born After 2004 in the United Kingdom. Pediatric Infect Dis J. 2021;40(6):590-6.
- Berry AA, Abu-Elyazeed R, Diaz-Perez C, Mufson MA, Harrison CJ, Leonardi M, et al. Two-year antibody persistence in children vaccinated at 12–15 months with a measles-mumps-rubella virus vaccine without human serum albumin. Hum Vacc Immunother. 2017;13(7):1516-22.
- Gothefors L, Bergström E, Backman M. Immunogenicity and Reactogenicity of a New Measles, Mumps and Rubella Vaccine When Administered as a Second Dose at 12 y of Age. Scand J Infect Dis. 2009;33(7):545-9.
- Group M-S. Safety and immunogenicity of an upper-range release titer measles-mumps-rubella vaccine in children vaccinated at 12 to 15 months of age: a phase III, randomized study. Hum Vacc Immunother. 2018;14(12):2921-31.
- Mufson MA, Diaz C, Leonardi M, Harrison CJ, Grogg S, Carbayo A, et al. Safety and Immunogenicity of Human Serum Albumin-Free MMR Vaccine in US Children Aged 12–15 Months. J Pediatric Infect Dis Soc. 2015;4(4):339-48.
- Gatchalian S, Cordero-Yap L, Lu-Fong M, Soriano R, Ludan A, Chitour K, et al. A randomized comparative trial in order to assess the reactogenicity and immunogenicity of a new measles mumps rubella (MMR) vaccine when given as a first dose at 12-24 months of age. Southeast Asian J Tropical Medicine Public Heal. 1999;30(3):511-7.
- Group M-S. Immunogenicity and safety of measles-mumps-rubella vaccine at two different potency levels administered to healthy children aged 12-15 months: a phase III, randomized, non-inferiority trial. Vaccine. 2018;36(38):5781‐8.
- Group M-S. A second dose of a measles-mumps-rubella vaccine administered to healthy four-to-six-year-old children: a phase III, observer-blind, randomized, safety and immunogenicity study comparing GSK MMR and MMR II with and without DTaP-IPV and varicella vaccines co-administration. Hum Vaccin Immunother. 2019;15(4):786-99.
- Klein NP, Abu-Elyazeed R, Povey M, Parra MM, Diez-Domingo J, Ahonen A, et al. Immunogenicity and Safety of a Measles-Mumps-Rubella Vaccine Administered as a First Dose to Children Aged 12 to 15 Months: A Phase III, Randomized, Noninferiority, Lot-to-Lot Consistency Study. J Pediatric Infect Dis Soc. 2019;9(2):194-201.
- Lee C-Y, Tang R-B, Huang F-Y, Tang H, Huang L-M, Bock HL. A new measles mumps rubella (MMR) vaccine: a randomized comparative trial for assessing the reactogenicity and immunogenicity of three consecutive production lots and comparison with a widely used MMR vaccine in measles primed children. Int J Infect Dis. 2002;6(3):202-9.
- Lee H, Kim HW, Cho HK, Park EA, Choi KM, Kim KH. Reappraisal of MMR vaccines currently used in Korea. Pediatr Int. 2011;53(3):374-80.
- Usonis V, Bakasenas V, Chitour K, Clemens R. Comparative study of reactogenicity and immunogenicity of new and established measles, mumps and rubella vaccines in healthy children. Infection. 1998;26(4):222-6.
- Usonis V, Bakasenas V, Denis M. Neutralization Activity and Persistence of Antibodies Induced in Response to Vaccination with a Novel Mumps Strain, RIT 4385. Infection. 2001;29(3):159-62.
- Usonis V, Bakasenas V, Kaufhold A, Chitour K, Clemens R. Reactogenicity and immunogenicity of a new live attenuated combined measles, mumps and rubella vaccine in healthy children. Pediatric Infect Dis J. 1999;18(1):42-8.
- Global Advisory Committee on Vaccine Safety, 11–12 June 2003. Weekly Epidemiological Record. 2003 2003;78(32):277-84.
- Farrington P, Rush M, Miller E, Pugh S, Colville A, Flower A, et al. A new method for active surveillance of adverse events from diphtheria/tetanus/pertussis and measles/mumps/rubella vaccines. Lancet. 1995;345(8949):567-9.
- France EK, Glanz J, Xu S, Hambidge S, Yamasaki K, Black SB, et al. Risk of Immune Thrombocytopenic Purpura After Measles-Mumps-Rubella Immunization in Children. Pediatrics. 2008;121(3):e687-e92.
- Miller E, Andrews N, Stowe J, Grant A, Waight P, Taylor B. Risks of Convulsion and Aseptic Meningitis following Measles-Mumps-Rubella Vaccination in the United Kingdom. Am J Epidemiol. 2007;165(6):704-9.
- O’Leary ST, Glanz JM, McClure DL, Akhtar A, Daley MF, Nakasato C, et al. The Risk of Immune Thrombocytopenic Purpura After Vaccination in Children and Adolescents. Pediatrics. 2012;129(2):248-55.
- Perez-Vilar S, Weibel D, Sturkenboom M, Black S, Maure C, Castro JL, et al. Enhancing global vaccine pharmacovigilance: Proof-of-concept study on aseptic meningitis and immune thrombocytopenic purpura following measles-mumps containing vaccination. Vaccine. 2018;36(3):347-54.
- Pietrantonj CD, Rivetti A, Marchione P, Debalini MG, Demicheli V. Vaccines for measles, mumps, rubella, and varicella in children. Cochrane Db Syst Rev. 2021;2021(11):CD004407.
- Package Insert: PRIORIX (Measles, Mumps, and Rubella Vaccine, Live). FDA; [cited 2022 8/1/2022]; Available from: https://www.fda.gov/media/158941/download.
- Package Insert: M-M-R® II (Measles, Mumps, and Rubella Virus Vaccine Live). FDA; [cited 2022 8/1/2022]; Available from: https://www.fda.gov/media/75191/download.
- Control CfD, Prevention. Shortage of varicella and measles, mumps and rubella vaccines and interim recommendations from the Advisory Committee on Immunization Practices. Mmwr Morbidity Mortal Wkly Rep. 2002;51(9):190-1.