At a glance
- CDC has authorities to implement regulations focused on protecting America from certain preventable diseases and health threats.
- CDC and other agencies implement public health laws passed by Congress through Federal regulations.
- Federal agencies put laws into action by developing regulations—also known as “rules”—through a process called “rulemaking.”
- Federal rules give the public details or specific requirements of how the law will be applied.
What is a regulation?
A regulation, or rule, is a general statement issued by an agency, board, or commission that has the force and effect of law. Congress often grants agencies the authority to issue regulations through legislation. Federal regulations specify the details and requirements necessary to implement and to enforce the legislation enacted by Congress.
The rulemaking process
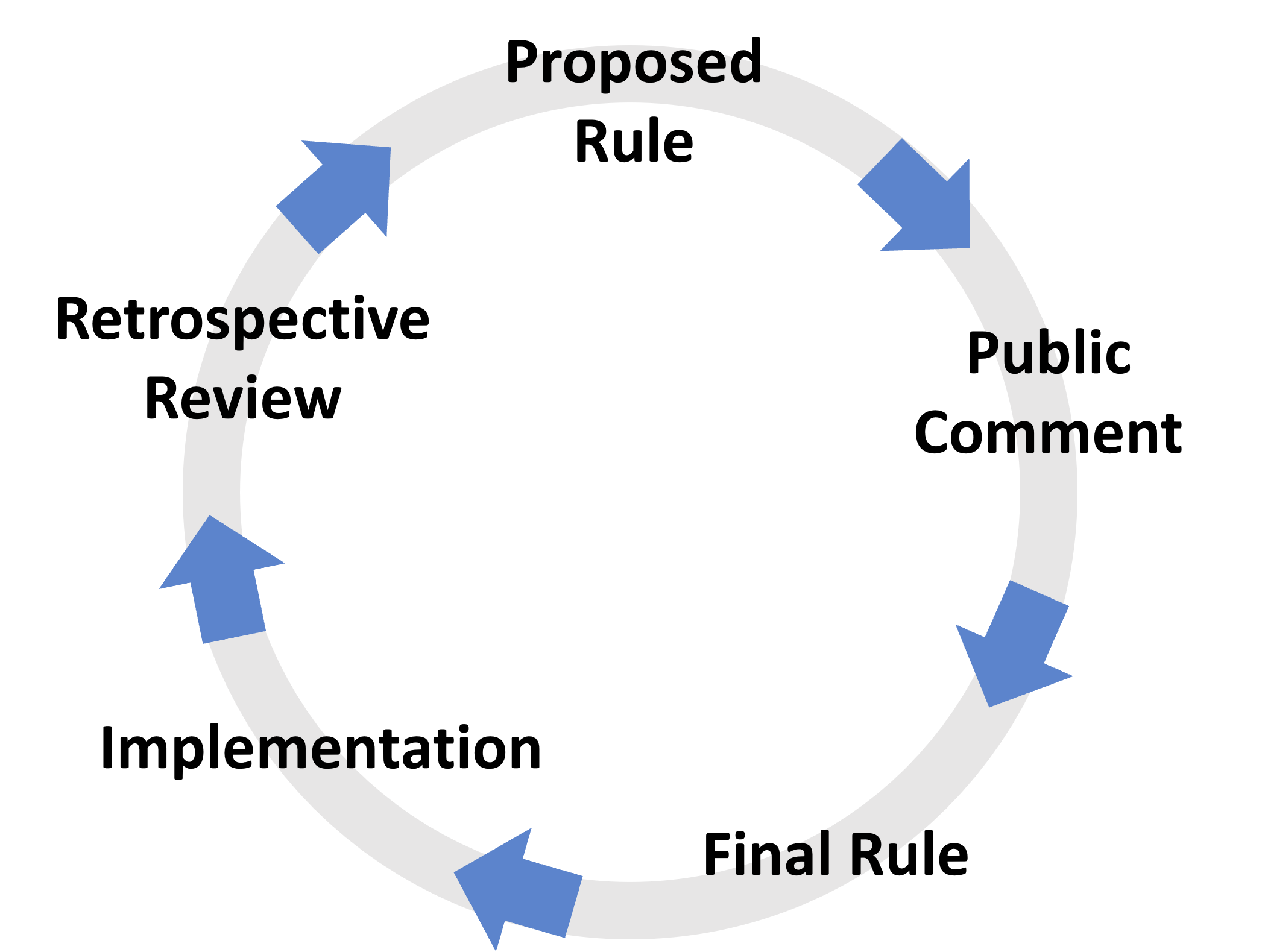
- Federal regulations are created through a process known as rulemaking, which is governed by the Administrative Procedure Act (APA) (5 U.S.C. Chapter 5).
- Once an agency decides that a regulatory action is necessary or appropriate to address problems or accomplish goals, it develops and typically publishes a proposed rule in the Federal Register, soliciting comments from the public on the regulatory proposal.
- The Federal Register is the official daily publication for agency rules, proposed rules, and notices of Federal agencies and organizations, as well as for Executive Orders and other presidential documents.
- The Federal Register is the official daily publication for agency rules, proposed rules, and notices of Federal agencies and organizations, as well as for Executive Orders and other presidential documents.
- After the agency considers this public feedback and makes changes where appropriate, it then publishes a final rule in the Federal Register with a specific date upon which the rule becomes effective and enforceable.
- In issuing a final rule, the agency must describe and respond to the public comments it received.
- In issuing a final rule, the agency must describe and respond to the public comments it received.
- After a final rule is published, the implementation stage goes into effect for individuals and industries affected by the rule.
- Implementation often includes guidance documents–e.g., compliance materials and technical assistance manuals–that provide information regarding regulatory requirements.
- Implementation often includes guidance documents–e.g., compliance materials and technical assistance manuals–that provide information regarding regulatory requirements.
- Retrospective review is an ongoing process by which agencies assess existing rules and decide whether they need to be revisited.
How can you participate in CDC’s rulemaking process?
Federal rulemaking usually involves the notice-and-comment process described above, in which rules are published in the Federal Register for public comment over a specified time. By commenting on proposed rules to improve them, the public plays an important role in the rulemaking process. You can provide your comments by going to the federal government's regulations website.
For a list of regulations CDC is currently working on, please see the Unified Agenda of Federal Regulatory and Deregulatory Actions. The Unified Agenda, published in the spring and fall of each year, provides information about regulations that the federal government is considering or reviewing. CDC's regulations are published in the Code of Federal Regulations under Title 42—Public Health. See CDC Regulations by Topic for links to CDC's regulatory text.
Contacts
Email CDCRegulations@cdc.gov with questions, comments, or suggestions on how to improve CDC's regulations.
