Key points
- There is a wide range of electromagnetic radiation in nature, and visible light is one example.
- Radiation with the highest energy includes forms like ultraviolet radiation, x-rays, and gamma rays.
- X-rays and gamma rays can remove electrons and cause the atom to become ionized.

Overview
The most common form of radiation we are all familiar with is visible light. Light is energy that originates from a source and travels through space at the speed of...light! It has a particular frequency that defines its energy.
We can detect this visible radiation with our eyes. The only difference between various colors of light is in their wavelength and frequency or in other words in their energy. Red light, for example, has less energy than purple light.
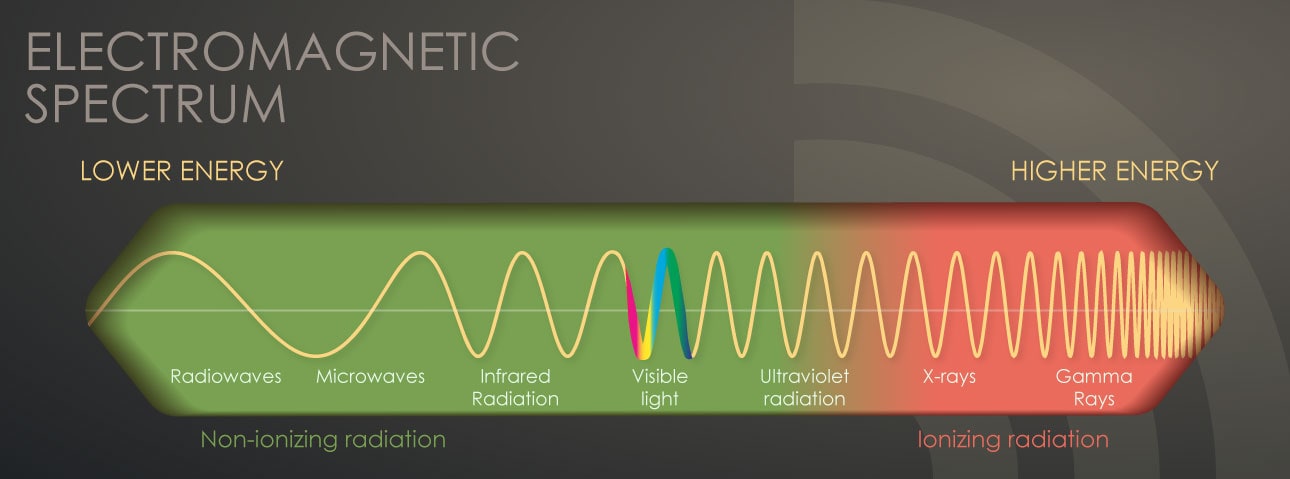
The visible part of the spectrum is only a tiny part of this wide range of energies.
As we move lower in frequency from red light, there are other familiar forms of electromagnetic radiation:
- Infrared
- Microwaves
- Signals from our cell phones
- Radio waves
These are all forms of radiation that are invisible to our eyes and that have less energy than visible light or light of different colors.
As we move up (higher) in frequency from purple light, there are:
These are all forms of radiation with energies much higher than visible light.
X-rays and gamma rays have enough energy that during interaction with atoms, they can remove electrons. This causes the atom to become charged or ionized. That's why we refer to this as ionizing radiation. When most people talk about radiation, they are referring to ionizing radiation.
Ionization is a unique property that other forms of radiation at lower frequencies do not have.
Resources
Glossary
Frequency - How rapidly waves move or 'oscillate' up and down. The lower the frequency of the radiation, the lower its energy.
Ionizing radiation - Any radiation capable of displacing electrons from atoms, thereby producing ions. High doses of ionizing radiation may produce severe skin or tissue damage.
Ionization - The process of adding one or more electrons or removing one or more electrons. Very high temperatures, electrical discharges, or nuclear radiation can cause ionization.
Radiation - Energy in the form of particles or waves moving through space or matter. Familiar radiations are heat, light, radio waves, and microwaves. Ionizing radiation is a very high-energy form of electromagnetic radiation.
Wavelength – Distance covered by one complete cycle of the electromagnetic wave. In other words, the distance from one peak to another peak or from trough to trough in one wave. The longer the wavelength of the radiation, the lower its energy.
