DPDM NewsBites: December 2022

Pictured: CDC staff reviewing potential breeding sites of the blackfly, the vector for Onchocerca volvulus, along the White Volta River and tributaries in Ghana.
The Division of Parasitic Diseases and Malaria (DPDM) is guided by the same focus that drives all of CDC’s activities: use science and innovation to prevent, detect, and respond to public health threats. DPDM addresses diseases caused by parasites, including malaria, cyclosporiasis, Chagas disease, and neglected tropical diseases (NTDs), among dozens of others.
We continue to advance forward towards three priority goals to help save and improve lives by addressing malaria and other parasitic diseases in the U.S. and around the world. Here’s a look back at some of the important progress we’ve made this past year.
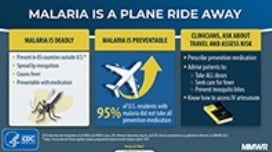
There were 1,823 confirmed malaria infections in the United States in 2018, mostly people who traveled to areas with ongoing malaria transmission. Of the U.S. civilian patients who reported reason for travel, 77% were visiting friends and relatives (VFR). As in previous years, approximately 60% of all cases imported into the U.S. were attributable to persons who had traveled in West Africa, and two thirds of VFR travelers went to West Africa. The number of cases diagnosed in the United States and its territories has been increasing since the mid-1970s alongside the rise in international travel.
Malaria infections can be fatal if not diagnosed and treated promptly with appropriate antimalarial medications. In 2018, 251 of the reported malaria cases (13.8%) were severe. Having a plan for rapid diagnosis and treatment of malaria within 24 hours ensures that hospitals give patients with malaria the best chance of recovery.
Artesunate for InjectionTM—the first-line drug for treatment of severe malaria in the United States—is now manufactured, distributed, and commercially available in the United States. CDC discontinued its distribution of iintravenous artesunate for treatment of severe malaria in the U.S. on September 30, 2022. DPDM will continue to support the appropriate diagnosis and treatment of malaria in the U.S. through its ongoing 24/7/365 activities such as providing guidance and education on malaria.
Chagas disease is caused by the parasite, Trypanosoma cruzi, which is found only in the Americas and is primarily transmitted to animals and people by triatomine bugs in rural areas of Latin America where poverty is widespread. Chagas disease can also be transmitted via blood transfusion, organ transplantation, and mother-to-baby. An estimated 300 babies are born with Chagas disease in the U.S. every year and most are undiagnosed. If left untreated, Chagas disease can cause heart failure, stroke, and even death.
Over the past several years, CDC has funded three academic partners to develop new strategies, educational tools, materials, and guidelines to improve awareness and prevention of Chagas disease. As part of these efforts, this year the Chagas Disease ECHO program organized three sessions on Chagas disease, and One Health and Einstein College of Medicine ran a medical education webinar series on immigrant health including Chagas disease. DPDM collaborator, Dr. Rachel Marcus, presented a webinar on the cardiac manifestations of Chagas disease to help improve management of patients with Chagasic cardiomyopathy.
Supporting Afghan evacuees
The U.S Government’s Operation Allies Welcome (OAW) was activated from August 2021 to March 2022 to help evacuees from Afghanistan enter the United States. Multiple agencies welcomed more than 82,000 evacuees to help them safely resettle in US communities. As part of CDC’s response, DPDM provided technical assistance related to parasitic diseases of concern, including malaria and leishmaniasis. Leishmaniasis is an NTD that is endemic in Afghanistan and that can cause significant skin disease (cutaneous leishmaniasis) or organ dysfunction and death (visceral leishmaniasis). For both malaria and leishmaniasis, DPDM staff drafted health communication resource materials and partnered with colleagues at the University of Minnesota, the Uniformed Services University of the Health Sciences, and Walter Reed Army Institute of Research on a webinar educating health providers in diagnosis and management. DPDM also provided information for health providers on management of scabies and lice.
A recurring summertime foe
The summer season, particularly May through September, often brings a rise in cyclosporiasis in the United States. Cyclosporiasis is a diarrheal illness caused by a microscopic parasite. People can become infected by consuming contaminated food or water. The year 2022 was no exception, as multiple outbreaks of cyclosporiasis cases associated with different points of service were investigated by state public health authorities, CDC, and FDA. Overall, 1,129 laboratory laboratory-confirmed cases were reported to CDC by 34 jurisdictions.
To help combat the Cyclospora parasite, DPDM has been using advanced molecular detection (AMD) methods to develop DNA fingerprinting to help distinguish among different strains of the parasite that cause cyclosporiasis.
This summer, during a cyclosporiasis investigation, CDC used its novel genotyping tool to clarify relationships among clusters of cyclosporiasis illnesses in three states, and this information helped inform FDA’s traceback investigations. FDA was able to trace clusters in two different states because of a genetic link, even though the suspect foods in each cluster were different; they turned out to be produced by the same company. Additionally, FDA expanded its traceback investigation of a cluster in another state to include two additional healthcare facilities because of a genetic link among patients at those other facilities, even though no suspect food could be identified from those secondary clusters. Investigations showed that the same product was supplied to all the facilities. These clusters would not have been linked by traditional epi methods alone.
Female Anopheles stephensi mosquito
The growing threat from an invasive mosquito
In 2012, routine public health tracking activities first detected that a mosquito species called Anopheles stephensi (An. stephensi) had found its way from its native habitat in Southern Asia to Africa. Since first being identified in Djibouti, it has been identified in neighboring Ethiopia (2016), Sudan (2019), and Somalia (2019). Most recently, in June 2022, Nigeria confirmed the presence of An. stephensi mosquitoes, making it the first country outside of the Horn of Africa. Nigeria accounts for nearly one third of all global malaria cases and nearly one quarter of global malaria deaths. DPDM and the U.S. President’s Malaria Initiative (PMI) Anopheles stephensi Task Force are supporting Nigerian activities to monitor and respond to this emerging threat.
Recent findings from Ethiopia (supported by PMI and CDC activities) linked invasive An. stephensi to increases in urban malaria in Dire Dawa, Ethiopia. These findings provide evidence needed to confirm that this species does present a threat to malaria control and elimination in Africa and may reverse gains made in recent decades. Modeling estimates have shown that if An. stephensi continues to spread it may result in an additional 126 million people at risk of malaria in Africa, particularly in urban areas. Recognizing this emerging global threat, the World Health Organization recently launched the WHO Initiative to stop the spread of Anopheles stephensi in Africa.
DPDM is providing support to the West African Aedes Surveillance Network (WAASuN) to train entomologists on morphological identification of An. stephensi. DPDM also collaborated with Baylor University in Texas to study population genetics to identify where An. stephensi is coming from, how it is spreading, and how long it has been on the continent.
You can read more about this invasive mosquito threat to malaria in recent coverage from Science magazine and National Public Radio (NPR).

Clinical lab at monoclonal trial site.
New hopes to protect against malaria
Malaria caused approximately 247 million illnesses and approximately 619,000 deaths globally in 2021. Sub-Saharan Africa accounts for 95% of all malaria cases and 96% of deaths globally, with 80% of these deaths among children <5 years old. The world’s first malaria vaccine, RTS,S/AS01, was recently recommended for widespread use in areas with moderate to high malaria transmission. The vaccine carries the potential to significantly reduce illness and death from malaria in Africa but also has a complex 4-dose schedule and approximately 40% efficacy over 4 years. Moreover, vaccine supply remains very limited. DPDM is currently working on a study to evaluate the effectiveness of fractionaldose versus full-dose regimens of the RTS,S/AS01 malaria vaccine to understand if the currently available supply can be stretched to protect more children.
Given the very limited availability of the RTS,S vaccine, there remains an urgent need for safe and more effective malaria vaccines and other novel interventions, such as monoclonal antibodies. Monoclonal antibodies are proteins made in laboratories that bind to foreign materials like pathogens in the body in order to destroy them.
In June 2022, DPDM partnered with the CDC Foundation, the Vaccine Research Center of the National Institute of Allergy and Infectious Diseases (NIAID; part of the National Institutes of Health (NIH)), and the Bill & Melinda Gates Foundation to develop a new clinical trial with the Kenya Medical Research Institute (KEMRI) and the Liverpool School of Tropical Medicine (LSTM) in western Kenya. The trial seeks to evaluate the safety and efficacy of L9LS, a human monoclonal antibody against Plasmodium falciparum malaria in Western Kenya. An intervention like this that could prevent malaria in children for a full 12 months in settings of perennial malaria transmission, such as Kenya, holds potential to substantially reduce malaria morbidity and mortality.
The first phase of the trial began in October 2022 with the initial doses of L9LS administered to participating children aged 5-10 years old followed by children 6-59 months. The children will be monitored closely for any side effects or adverse reactions over the next three months; initial follow-up data demonstrated the product to be very safe. The second phase, to assess the efficacy of the monoclonal antibody over one year among children 5-59 months will begin in early January.
Helping to ensure provision of life-saving interventions against malaria in the face in COVID-19
As the world reeled from the numbers of cases and deaths due to COVID-19 in 2020, hundreds of thousands of families mourned losses from another killer disease – malaria. CDC quickly leveraged its in-country expertise, programs, and partnerships in 4 African countries (Kenya, Nigeria, Malawi, Uganda) and Haiti, to improve understanding of how to safely deliver malaria control interventions in the context of the COVID-19 pandemic.
For example, when COVID-19 hit Uganda, PMI funded a survey to understand challenges Community Health Workers (CHWs) faced in their malaria prevention work. Results showed that only slightly more than half of them had access to a functional handwashing station, fewer than a third thought COVID-19 was a serious risk, and most were not following COVID-19 procedures they had been trained on.
CDC, in collaboration with the Uganda Ministry of Health and local partners responded by developing a program to address gaps, helping train more than 900 CHW and providing a year’s supply of hand sanitizer, personal protective equipment, rapid tests, antibiotics, antimalarials, and other treatments. Albert Anyaja, a Community Health Worker, shared, “When COVID-19 started, mothers feared bringing their children here, even some of my colleagues abandoned work because of fear of getting COVID-19 from people bringing their children for treatment. When we received training and protective things like a handwash station, gloves, face masks, and liquid soap from IDI, we started telling people. They started coming in large numbers. I treated many children with fever.”
While a seemingly simple intervention in terms of providing basic supplies for hand washing and other needs, these efforts filled a critical gap and helped to ensure that routine malaria and other critical health services could continue safely.

Haitian children await MDA for lymphatic finariasis.
Striving to eliminate lymphatic filariasis “close” to home
Lymphatic filariasis (LF) is a debilitating NTD that has been targeted for elimination as a public health problem globally. DPDM, together with the CDC Foundation and the Pacific Island Health Officers Association, has been providing financial and technical assistance to the Department of Health in American Samoa (ASDOH), the last remaining area within the U.S. where LF still poses a risk. ASDOH aims to eliminate the disease using the WHO-recommended triple drug therapy (ivermectin, diethylcarbamazine, albendazole), which CDC helped evaluate for safety and effectiveness. American Samoa was among the first adopters of triple drug therapy, along with four other Pacific Island nations.
With CDC support, treatment coverage in American Samoa had surpassed the WHO recommended targets prior to the 3rd round of triple drug therapy. ASDOH carried out a third round of triple drug mass drug administration (MDA) from September 2021 – January 2022 and an impact assessment in October – November 2022 with technical assistance from DPDM. Results of the survey will determine if MDA can be safely stopped.
LF also continues to pose a health risk to the people of Haiti. DPDM has also been providing ongoing technical support to the Haitian Ministry of Public Health and Population (MSPP) and partners to achieve the elimination of LF as a public health problem.
DPDM has helped to assess the cost effectiveness of delivering MDA door-to-door vs. through community distribution posts in the commune of Milot. DPDM worked with several internal and external partners to determine the effectiveness and community acceptability of delivering medications at home, as well as identify any challenges that may arise by using this strategy. These results are being used as part of broad efforts to improve medication delivery strategies to help communities meet treatment targets throughout Haiti. DPDM also aims to expand Haiti’s capacity to identify people who continue to harbor infection despite MDA (most likely because they are not participating,) so that they can be treated outside of MDA. We also want to determine the burden of people with LF-related morbidity so that they can receive care.
Together with partners, the National Programme for the Elimination of Malaria and Lymphatic Filariasis of the MSPP will officially launch the 2023 MDA campaign starting in three communes of the North region with more than 303,000 at risk people, using triple drug therapy. The main objective of the 2023 campaigns is provide MDA to approximately 850,000 people in 10 of the 18 municipalities in the North, North-West, and West regions with a high prevalence of disease.

Pictured: Filter paper disks with blood spots drying on kebab skewers and pencils. Dried blood spots will be used later for Ov-16 analyses for onchocerciasis.
Knowing when to stop (treatment)
Onchocerciasis, or river blindness, is an NTD that has been targeted for elimination of transmission. The WHO guidelines require country national onchocerciasis elimination programs to demonstrate that they have achieved specific milestones in humans and in the blackfly vector that helps spread the parasite. For the milestone in humans, programs must demonstrate that fewer than 1 in 1000 children have evidence of exposure using an OV-16 blood test prior to stopping MDA. This threshold may be too conservative, preventing programs that may have already interrupted transmission from stopping MDA. Additionally, it is challenging to confidently measure 0.1% using the currently available diagnostic tests.
Modeling suggests that a rate of even 1-2% (or higher) could be consistent with interruption of transmission.
DPDM is engaged in studies to demonstrate that higher thresholds may be used. The studies involve a series of entomologic and epidemiologic surveys at different timepoints and supporting national public health laboratories to run the needed diagnostic testing. If results continue to suggest an absence of transmission, this could provide evidence needed to demonstrate that the higher threshold level is consistent with interruption of transmission of infection. The studies are currently being conducted in Tanzania and Ghana, with an additional country to be added. DPDM has also provided advice to the Task Force for Global Health as it implements similar studies in two additional countries.
Helping get the “most bang for the buck”
CDC has developed and validated a multiplex immunoassay that can detect antibodies to more than 30 different viral, bacterial, and parasitic disease agents from just a single small blood sample. This test provides a more cost-effective approach to obtain critical public health information, as most surveillance costs are related to sample collection. Since 2012, CDC has worked with partners in over a dozen countries to leverage disease-specific population-based serosurveys, by testing for exposure to additional diseases of public health interest using the multiplex immunoassay.
Since 2015, DPDM has been working with the Pan American Health Organization (PAHO) and 5 partner countries to utilize multiplex immunoassays for integrated serosurveillance, including working with Mexico and Paraguay, to design and implement integrated serosurveillance with testing in their national laboratories. In September 2022, PAHO published their toolkit for integrated serosurveillance in the Americas, a guide for program managers, based on these experiences, as they move into the next phase of this initiative.
DPDM continues to support work started in 2019 at the National Reference Laboratory (NRL) in Nigeria, in which specimens collected during the 2018 Nigeria AIDS Indicator and Impact Survey were tested for antibodies to 19 different pathogens. These data have had wide-ranging public health impact, from informing vaccination campaigns in early 2020 to identifying new risk factors for the parasitic disease schistosomiasis. NRL is building on this foundation to conduct COVID-19 serosurveys and is planning to be a regional center for integrated serosurveillance.
Higher-Dose Primaquine to Prevent Relapse of Plasmodium vivax Malaria. New England Journal of Medicine.
March 2022.
Proactive community case management decreased malaria prevalence in rural Madagascar: results from acluster randomized trial. BMC Medicine. October 2022.
Public Health Surveillance and Reporting for Human Toxoplasmosis — Six States, 2021. Morbidity and Mortality
Weekly Report. July 15, 2022.
Progress Toward Global Eradication of Dracunculiasis — Worldwide, January 2021–June 2022. Morbidity and
Mortality Weekly Report. November 25, 2022.